| General Information of Drug (ID:
DR1792) |
| Drug Name |
R-1439
|
| Synonyms |
Aleglitazar; Aleglitazar (USAN); Aleglitazar [USAN:INN]; R-1439; RG-1439; RO-0728804; (2S)-2-methoxy-3-[4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]-1-benzothiophen-7-yl]propanoic acid; (2S)-2-methoxy-3-{4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]-1-benzothiophen-7-yl}propanoic acid; (S)-2-methoxy-3-[4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-ethoxy]-benzo[b]thiophen-7-yl}-propionic acid; 41T4OAG59U; 475479-34-6; C24H23NO5S; CHEMBL519504; R1439; RO7; UNII-41T4OAG59U
|
| Indication |
Diabetes mellitus
[ICD11: 5A10]
|
Discontinued
|
[1]
|
| Structure |
|
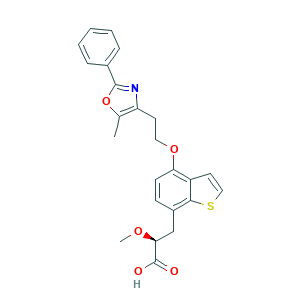 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
437.5 |
Topological Polar Surface Area |
110 |
| Heavy Atom Count |
31 |
Rotatable Bond Count |
9 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 10274777
- PubChem SID
-
15279780
; 22652721
; 35445984
; 74374212
; 74374213
; 74671516
; 96025528
; 99444954
; 103652550
; 104120360
; 126665625
; 134339048
; 135216342
; 136349978
; 137156430
; 141536129
; 160969520
; 163620807
; 163686133
; 170501031
; 175427152
; 176250129
; 178103977
; 185988952
; 198964037
; 215784940
; 223535497
; 223670924
; 223800328
; 226798862
; 241083923
; 245949369
; 252477574
- CAS Number
-
- TTD Drug ID
- D0B9EN
- Formula
- C24H23NO5S
- Canonical SMILES
- CC1=C(N=C(O1)C2=CC=CC=C2)CCOC3=C4C=CSC4=C(C=C3)CC(C(=O)O)OC
- InChI
- 1S/C24H23NO5S/c1-15-19(25-23(30-15)16-6-4-3-5-7-16)10-12-29-20-9-8-17(14-21(28-2)24(26)27)22-18(20)11-13-31-22/h3-9,11,13,21H,10,12,14H2,1-2H3,(H,26,27)/t21-/m0/s1
- InChIKey
- DAYKLWSKQJBGCS-NRFANRHFSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


