| General Information of Drug (ID:
DR1805) |
| Drug Name |
HSP-990
|
| Synonyms |
NVP-HSP-990; NVP-HSP990; NVP-HSP990 (HSP990); NVP-HSP990(HSP990); NVP-HSP990,HSP990; SB16642; SCHEMBL1351184; ZINC60329723; (7r)-2-Amino-7-[4-Fluoro-2-(6-Methoxypyridin-2-Yl)phenyl]-4-Methyl-7,8-Dihydropyrido[4,3-D]pyrimidin-5(6h)-One; 2608AH; 4u93; 934343-74-5; AOB87737; BCP9001013; BDBM50031735; CHEMBL3360305; CS-3941; DTXSID50239429; E0WBA7B62L; EX-A1560; HSP-990; HSP990; HSP990 (NVP-HSP); HSP990 (NVP-HSP990); HSP990, NVP-HSP990; UNII-E0WBA7B62L; hsp990-nvp-hsp990; s7097
|
| Indication |
Breast cancer
[ICD11: 2C60]
|
Phase 1
|
[1]
|
| Structure |
|
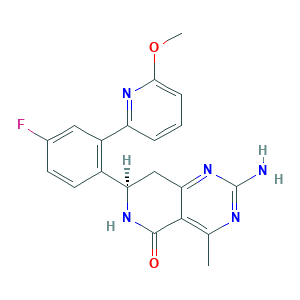 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
379.4 |
Topological Polar Surface Area |
103 |
| Heavy Atom Count |
28 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 46216556
- PubChem SID
-
96070909
; 120480897
; 136920357
; 136946398
; 137037402
; 140563242
; 144116301
; 172650605
; 186000974
; 198968137
; 211034684
; 223367201
; 227570856
; 244872166
; 249617120
; 249814491
; 252451799
; 252472803
- CAS Number
-
- TTD Drug ID
- D0K5BP
- Formula
- C20H18FN5O2
- Canonical SMILES
- CC1=C2C(=NC(=N1)N)CC(NC2=O)C3=C(C=C(C=C3)F)C4=NC(=CC=C4)OC
- InChI
- 1S/C20H18FN5O2/c1-10-18-16(26-20(22)23-10)9-15(25-19(18)27)12-7-6-11(21)8-13(12)14-4-3-5-17(24-14)28-2/h3-8,15H,9H2,1-2H3,(H,25,27)(H2,22,23,26)/t15-/m1/s1
- InChIKey
- WSMQUUGTQYPVPD-OAHLLOKOSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


