Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1815) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
ANW-60941
|
|||||
| Synonyms |
Dovitinib Lactate Hydrate; Dovitinib lactate; Dovitinib lactate (USAN); Dovitinib lactate [USAN]; Dovitinib lactate hydrate (JAN); Dovitinib lactate monohydrate; Dovitinib lactate(TKI258); SCHEMBL253994; TKI 258; TKI258; dovitinib-tki258-lactate; 4-Amino-5-fluoro-3-(6-(4-methylpiperazin-1-yl)-1H-benzo[d]imidazol-2-yl)quinolin-2(1H)-one 2-hydroxy; 4-Amino-5-fluoro-3-(6-(4-methylpiperazin-1-yl)-1H-benzo[d]imidazol-2-yl)quinolin-2(1H)-one 2-hydroxypropanoate hydrate; 915769-50-5; AN-564; ANW-60941; CTK8B8638; EX-A1642
|
|||||
| Indication | Uterine cancer [ICD11: 2C76] | Phase 2 | [1] | |||
| Structure |
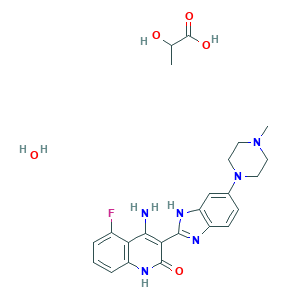 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 500.5 | Topological Polar Surface Area | 149 | ||
| Heavy Atom Count | 36 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 6 | Hydrogen Bond Acceptor Count | 10 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

