| General Information of Drug (ID:
DR1848) |
| Drug Name |
TAK-652
|
| Synonyms |
Cenicriviroc; Cenicriviroc (USAN/INN); SB16976; SCHEMBL3157748; SCHEMBL3157768; TAK-652; TAK652; TBR-652; TBR652; 1-Benzazocine-5-carboxamide, 8-[4-(2-butoxyethoxy)phenyl]-1,2,3,4-tetrahydro-1-(2-methylpropyl)-N-[4-[[(1-propyl-1H-imidazol-5-yl)methyl]sulfinyl]phenyl]-, (5E)-; 15C116UA4Y; 497223-25-3; AS-35184; BDBM50422828; CHEMBL2110727; CS-6148; D09878; DB11758; EX-A1608; HY-14882; MFCD28502076; UNII-15C116UA4Y; UNII-15C116UA4Y component PNDKCRDVVKJPKG-WHERJAGFSA-N
|
| Indication |
Human immunodeficiency virus infection
[ICD11: 1C60]
|
Phase 2
|
[]
|
| Structure |
|
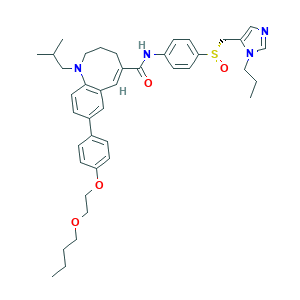 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
696.9 |
Topological Polar Surface Area |
105 |
| Heavy Atom Count |
50 |
Rotatable Bond Count |
17 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 11285792
- PubChem SID
-
3727064
; 16384602
; 23456613
; 42365221
; 74473324
; 135263513
; 135626631
; 137255612
; 163315791
; 164151703
; 164152753
; 198946191
; 198946193
; 210024091
; 229199515
; 229199534
- CAS Number
-
- TTD Drug ID
- D0YQ7U
- Formula
- C41H52N4O4S
- Canonical SMILES
- CCCCOCCOC1=CC=C(C=C1)C2=CC3=C(C=C2)N(CCCC(=C3)C(=O)NC4=CC=C(C=C4)S(=O)CC5=CN=CN5CCC)CC(C)C
- InChI
- 1S/C41H52N4O4S/c1-5-7-22-48-23-24-49-38-15-10-32(11-16-38)33-12-19-40-35(25-33)26-34(9-8-21-44(40)28-31(3)4)41(46)43-36-13-17-39(18-14-36)50(47)29-37-27-42-30-45(37)20-6-2/h10-19,25-27,30-31H,5-9,20-24,28-29H2,1-4H3,(H,43,46)/b34-26+/t50-/m0/s1
- InChIKey
- PNDKCRDVVKJPKG-WHERJAGFSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


