| General Information of Drug (ID:
DR1868) |
| Drug Name |
HSDB-1516
|
| Synonyms |
Benzofuroline; Benzyfuroline; Bioresmethrin (d trans isomer); Bioresmethrine; Chryson; Chrysron; Crossfire; ENT 27474; Enforcer; FMC 17370; For-syn; Isathrine; NIA 17370; NRDC 104; OMS-1206; Penick 1382; Penncapthrin; Premgard; Pyresthrin; Pyrethroids; Resbuthrin; Resmethrin [ANSI]; Resmethrine; Resmethrine [ISO-French]; Resmetrina [Portuguese]; S.B. Penick 1382; SB Pennick 1382; SBP-1382; Synthrin; d-trans-Resmethrin; resmethrin; 10453-86-8; 5-Benzylfurfuryl chrysanthemate; ARI-B; CCRIS 2501; Caswell No. 083E; EINECS 233-940-7; HSDB 1516
|
| Indication |
Allergy
[ICD11: 4A80]
|
Phase 2
|
[1]
|
| Structure |
|
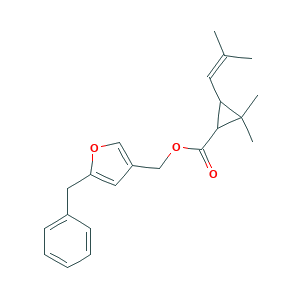 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
338.4 |
Topological Polar Surface Area |
39.4 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 5053
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09LEP
- Formula
- C22H26O3
- Canonical SMILES
- CC(=CC1C(C1(C)C)C(=O)OCC2=COC(=C2)CC3=CC=CC=C3)C
- InChI
- 1S/C22H26O3/c1-15(2)10-19-20(22(19,3)4)21(23)25-14-17-12-18(24-13-17)11-16-8-6-5-7-9-16/h5-10,12-13,19-20H,11,14H2,1-4H3
- InChIKey
- VEMKTZHHVJILDY-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


