| General Information of Drug (ID:
DR1879) |
| Drug Name |
BAY-8697661
|
| Synonyms |
Refametinib; Refametinib (RDEA119); JPX07AFM0N; RDEA 119; RDEA-119; RDEA119; Refametinib R enantiomer; Refametinib [INN]; SCHEMBL345333; (S)-N-(3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)-6-methoxyphenyl)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide; 3e8n; 923032-37-5; BAY 86-9766; BAY 86-97661; BAY 869766; BAY 8697661; BAY-869766; N-[3,4-difluoro-2-(2-fluoro-4-iodoanilino)-6-methoxyphenyl]-1-[(2S)-2,3-dihydroxypropyl]cyclopropane-1-sulfonamide; Refametinib (RDEA119, Bay 86-9766); UNII-JPX07AFM0N
|
| Indication |
Hepatocellular carcinoma
[ICD11: 2C12]
|
Phase 3
|
[1]
|
| Structure |
|
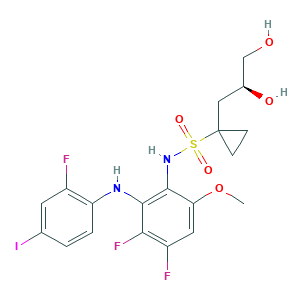 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
572.3 |
Topological Polar Surface Area |
116 |
| Heavy Atom Count |
31 |
Rotatable Bond Count |
9 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
10 |
| Cross-matching ID |
- PubChem CID
- 44182295
- PubChem SID
-
85177011
; 89943266
; 124899205
; 135264395
; 137275836
; 140908101
; 162037702
; 163563839
; 170482249
; 186000966
; 198043957
; 198960555
; 210275127
; 210280766
; 224705491
; 226680221
; 242059802
; 245093467
; 249565625
; 249623782
; 252216184
; 252451591
; 252470677
- CAS Number
-
- TTD Drug ID
- D03SKL
- Formula
- C19H20F3IN2O5S
- Canonical SMILES
- COC1=CC(=C(C(=C1NS(=O)(=O)C2(CC2)CC(CO)O)NC3=C(C=C(C=C3)I)F)F)F
- InChI
- 1S/C19H20F3IN2O5S/c1-30-15-7-13(21)16(22)18(24-14-3-2-10(23)6-12(14)20)17(15)25-31(28,29)19(4-5-19)8-11(27)9-26/h2-3,6-7,11,24-27H,4-5,8-9H2,1H3/t11-/m0/s1
- InChIKey
- RDSACQWTXKSHJT-NSHDSACASA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


