| General Information of Drug (ID:
DR1918) |
| Drug Name |
Cladribine
|
| Synonyms |
Chlorodeoxyadenosine; Cladarabine; Cladaribine; Cladribine; CldAdo; Leustat; Leustatin; Mylinax; RWJ 26251; RWJ-26251; (2R,3S,5R)-5-(6-amino-2-chloropurin-9-yl)-2-(hydroxymethyl)oxolan-3-ol; 2-CdA; 2-Chloro-2'-deoxy-beta-adenosine; 2-Chloro-2'-deoxyadenosine; 2-Chloro-6-amino-9-(2-deoxy-beta-D-erythropentofuranosyl)purine; 2-Chlorodeoxyadenosine; 2CdA; 2ClAdo; 4291-63-8; 47M74X9YT5; ADENOSINE, 2-CHLORO-2'-DEOXY-; BRN 0624220; CHEBI:567361; Litak; MFCD00153939; MLS000028377; NSC 105014; NSC 105014-F; SMR000058553; UNII-47M74X9YT5
|
| Indication |
Multiple myeloma
[ICD11: 2A83]
|
Approved
|
[1]
|
| Structure |
|
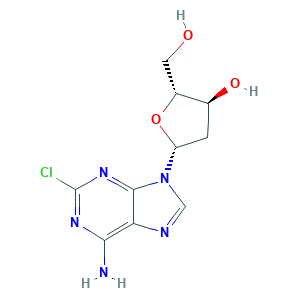 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
285.69 |
Topological Polar Surface Area |
119 |
| Heavy Atom Count |
19 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 20279
- PubChem SID
-
610461
; 855756
; 866156
; 7848433
; 7978577
; 7978957
; 8165006
; 14799875
; 14897807
; 26719669
; 26757803
; 29287787
; 46386544
; 46504588
; 48415788
; 49865077
; 49903916
; 49903918
; 50104045
; 50446026
; 56312468
; 56312469
; 56312470
; 56312853
; 56422184
; 57309572
; 57330593
; 71821359
; 87323981
; 92308638
; 92713835
; 99218181
; 99431527
; 99437023
; 103602769
; 104350170
; 118046705
; 124659094
; 124757075
; 124800053
; 124886798
; 124886799
; 125163879
; 126624559
; 126655830
; 128966217
; 131314655
; 134337856
; 134984314
; 135683425
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05GJW
- Formula
- C10H12ClN5O3
- Canonical SMILES
- C1C(C(OC1N2C=NC3=C(N=C(N=C32)Cl)N)CO)O
- InChI
- 1S/C10H12ClN5O3/c11-10-14-8(12)7-9(15-10)16(3-13-7)6-1-4(18)5(2-17)19-6/h3-6,17-18H,1-2H2,(H2,12,14,15)/t4-,5+,6+/m0/s1
- InChIKey
- PTOAARAWEBMLNO-KVQBGUIXSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


