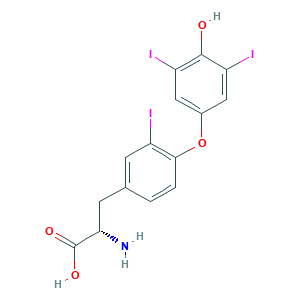
| Synonyms |
Reverse Tri-Iodothyronine; Triiodothyronine, reverse; (2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3-iodophenyl]propanoic acid; (S)-2-Amino-3-(4-(4-hydroxy-3,5-diiodophenoxy)-3-iodophenyl)propanoic acid; 3,3',5'-Triiodo-L-thyronine; 3,3',5'-Triiodothyronine; 5817-39-0; 3,3',5'-Triiodo-l-thyronine; REVERSE TRIIODOTHYRONINE; Reverse T3; 8NZ4Y08T96; CHEBI:11684; DSSTox_CID_26908; DSSTox_GSID_46908; DSSTox_RID_82006; L-Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3-iodo-; Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3-iodo-; UNII-8NZ4Y08T96
|
| Cross-matching ID |
- PubChem CID
- 644280
- ChEBI ID
-
- CAS Number
-
- Formula
- C15H12I3NO4
- Canonical SMILES
- C1=CC(=C(C=C1CC(C(=O)O)N)I)OC2=CC(=C(C(=C2)I)O)I
- InChI
- 1S/C15H12I3NO4/c16-9-3-7(4-12(19)15(21)22)1-2-13(9)23-8-5-10(17)14(20)11(18)6-8/h1-3,5-6,12,20H,4,19H2,(H,21,22)/t12-/m0/s1
- InChIKey
- HZCBWYNLGPIQRK-LBPRGKRZSA-N
|