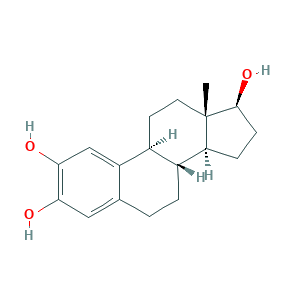
| Synonyms |
Estra-1,3,5(10)-triene-2,3,17beta-triol; MLS000069506; NSC 61711; 2-Hydroxyestradiol; AYU2L67YUU; DILDHNKDVHLEQB-XSSYPUMDSA-N; (17beta)-Estra-1,3,5(10)-triene-2,3,17-triol; 1,3,5(10)-estratriene-2,3,17Beta-triol; 2,3,17beta-Trihydroxy-1,3,5(10)-Estratriene; 2-Hydroxy-17; 2-Hydroxyestradiol-17beta; 2-OH-E2; 2-OH-Estradiol; 2-hydroxy-17beta-estradiol; 2-hydroxy-estradiol; 2bw7; 362-05-0; BRN 2219367; CCRIS 8709; CHEBI:28744; CHEMBL467987; ECS; ESTRA-1,3,5(10)-TRIENE-2,3,17-beta-TRIOL; SMR000058615; UNII-AYU2L67YUU
|
| Cross-matching ID |
- PubChem CID
- 247304
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00PZE
- Formula
- C18H24O3
- Canonical SMILES
- CC12CCC3C(C1CCC2O)CCC4=CC(=C(C=C34)O)O
- InChI
- 1S/C18H24O3/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-15(19)16(20)9-13(10)11/h8-9,11-12,14,17,19-21H,2-7H2,1H3/t11-,12+,14-,17-,18-/m0/s1
- InChIKey
- DILDHNKDVHLEQB-XSSYPUMDSA-N
|