| Synonyms |
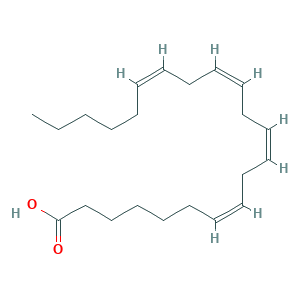
Adrenic acid; TWSWSIQAPQLDBP-DOFZRALJSA-N; (7Z,10Z,13Z,16Z)-Docosa-7,10,13,16-tetraenoic acid; 28874-58-0; 7,10,13,16-Docosatetraenoate; 7,10,13,16-Docosatetraenoic acid; 7,10,13,16-Docosatetraenoic acid, (all-Z)-; 7,10,13,16-docosatetraenoic acid, (7Z,10Z,13Z,16Z)-; 7Z,10Z,13Z,16Z-Docosatetraenoic acid; AC1NUZMR; Cis-7,10,13,16-docosatetraenoic acid; BSPBio_001499; CHEBI:53487; CHEMBL1491103; HMS1791K21; HMS1989K21; SCHEMBL19452; all-cis-7,10,13,16-Docosatetraenoic acid; all-cis-docosa-7,10,13,16-tetraenoic acid; cis-7,10,13,16-Docosatetraenoic acid
|
| Cross-matching ID |
- PubChem CID
- 5497181
- ChEBI ID
-
- CAS Number
-
- Formula
- C22H36O2
- Canonical SMILES
- CCCCCC=CCC=CCC=CCC=CCCCCCC(=O)O
- InChI
- 1S/C22H36O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-21H2,1H3,(H,23,24)/b7-6-,10-9-,13-12-,16-15-
- InChIKey
- TWSWSIQAPQLDBP-DOFZRALJSA-N
|