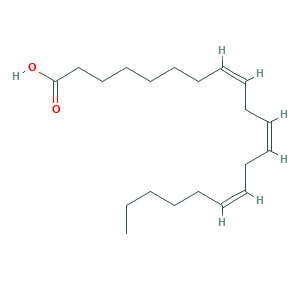
| Synonyms |
Bishomo-gamma-linolenic acid; Cis-8,11,14-eicosatrienoic acid; Dihomo-gamma-linolenic acid; Homo-gamma-linolenic acid; Ro 12-1989; cis-8,11,14-Eicosatrienoic Acid; gamma-Homolinolenic acid; (8Z,11Z,14Z)-Icosatrienoic acid; (8Z,11Z,14Z)-icosa-8,11,14-trienoic acid; (Z,Z,Z)-8,11,14-Eicosatrienoic acid; (Z,Z,Z)-8,11,14-Icosatrienoic acid; 1783-84-2; 8,11,14-Eicosatrienoate; 8,11,14-Eicosatrienoic acid; 8,11,14-Icosatrienoate; 8Z,11Z,14Z-eicosatrienoic acid; DGLA; UNII-FC398RK06S; all-cis-8,11,14-Eicosatrienoic acid; cis,cis,cis-8,11,14-Eicosatrienoic acid
|
| Cross-matching ID |
- PubChem CID
- 5280581
- PubChem SID
-
6112
; 841844
; 7850023
; 7888613
; 8616330
; 14849827
; 24894557
; 26754937
; 26754938
; 39289665
; 46508866
; 47364932
; 47885171
; 50110840
; 50834734
; 57357798
; 85787438
; 87322643
; 92309702
; 99300616
; 99302134
; 103636894
; 104046532
; 104115205
; 113853558
; 126524681
; 134222871
; 134338087
; 134978911
; 137205464
; 139646151
; 160963503
; 179150079
; 184568160
; 198986692
; 225022227
; 227115049
; 250134279
; 252368023
; 252455426
; 252457617
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0O1TC
- Formula
- C20H34O2
- Canonical SMILES
- CCCCCC=CCC=CCC=CCCCCCCC(=O)O
- InChI
- 1S/C20H34O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h6-7,9-10,12-13H,2-5,8,11,14-19H2,1H3,(H,21,22)/b7-6-,10-9-,13-12-
- InChIKey
- HOBAELRKJCKHQD-QNEBEIHSSA-N
|