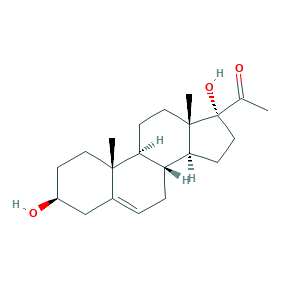
| Synonyms |
(3-beta)-3,17-Dihydroxypregn-5-en-20-one; 17-Hydroxy-delta5-pregnenolone; 17-Hydroxypregnenolone; 17-OH-pregnenolone; 17-alpha-Hydroxypregnenolone; 17a-Hydroxypregnenolone; 17alpha-Hydroxypregnenolone; 3-beta,17-Dihydroxypregn-5-en-20-one; 3-beta,17-alpha-Dihydroxypregn-5-en-20-one; 387-79-1; 3b,17-Dihydroxy-5-pregnen-20-one; 5-Pregnen-3beta,17alpha-diol-20-one; JERGUCIJOXJXHF-TVWVXWENSA-N; 77ME40334S; CHEBI:28750; CHEMBL408706; EINECS 206-862-6; 17 Alpha-Hydroxypregnenolone; Pregn-5-en-20-one, 3-beta,17-dihydroxy-; UNII-77ME40334S
|
| Cross-matching ID |
- PubChem CID
- 91451
- PubChem SID
-
841443
; 855825
; 10224803
; 14875321
; 17425085
; 24702314
; 44422400
; 50019734
; 53790772
; 57334862
; 71831438
; 76250425
; 92298057
; 93167067
; 103566225
; 104404818
; 123080711
; 124800596
; 125309465
; 126523206
; 128264115
; 135048384
; 137236028
; 164152383
; 164760906
; 178101796
; 179505061
; 184545691
; 221121815
; 227022703
; 249857761
; 249910018
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06MRM
- Formula
- C21H32O3
- Canonical SMILES
- CC(=O)C1(CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)O
- InChI
- 1S/C21H32O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h4,15-18,23-24H,5-12H2,1-3H3/t15-,16+,17-,18-,19-,20-,21-/m0/s1
- InChIKey
- JERGUCIJOXJXHF-TVWVXWENSA-N
|