| General Information of Drug (ID:
DR2122) |
| Drug Name |
Phosphatidylcholine
|
| Synonyms |
Phosphatidylcholine; ZINC32792133; (7s)-4-Hydroxy-N,N,N-Trimethyl-9-Oxo-7-[(Palmitoyloxy)methyl]-3,5,8-Trioxa-4-Phosphahexacosan-1-Aminium 4-Oxide; PHOSPHATIDYLCHOLINE; 1-PALMITOYL-2-STEAROYL-PC |
| Indication |
Discovery agent
|
Investigative
|
[1]
|
| Structure |
|
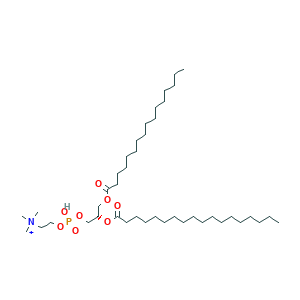 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
763.1 |
Topological Polar Surface Area |
108 |
| Heavy Atom Count |
52 |
Rotatable Bond Count |
42 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 14389436
- Formula
- C42H85NO8P+
- Canonical SMILES
- CCCCCCCCCCCCCCCCCC(=O)OC(COC(=O)CCCCCCCCCCCCCCC)COP(=O)(O)OCC[N+](C)(C)C
- InChI
- 1S/C42H84NO8P/c1-6-8-10-12-14-16-18-20-21-23-25-27-29-31-33-35-42(45)51-40(39-50-52(46,47)49-37-36-43(3,4)5)38-48-41(44)34-32-30-28-26-24-22-19-17-15-13-11-9-7-2/h40H,6-39H2,1-5H3/p+1/t40-/m0/s1
- InChIKey
- PZNPLUBHRSSFHT-FAIXQHPJSA-O
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


