| Cross-matching ID |
- PubChem CID
- 18068
- PubChem SID
-
585432
; 820510
; 822374
; 828118
; 841579
; 7887809
; 8010104
; 8163516
; 10322886
; 11532691
; 16017796
; 26702315
; 29285756
; 47169957
; 50080759
; 57329955
; 77612459
; 85198044
; 85198047
; 85856214
; 85856216
; 90344356
; 99455629
; 103516228
; 104344147
; 126523149
; 134228784
; 134356834
; 134356838
; 134356849
; 134356851
; 134419202
; 134982455
; 135651585
; 137255161
; 152186850
; 152255822
; 160645661
; 160963343
; 164161931
; 164161944
; 164194277
; 223730435
; 223730438
; 227723596
; 252452260
; 252452263
; 252452268
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0K7WW
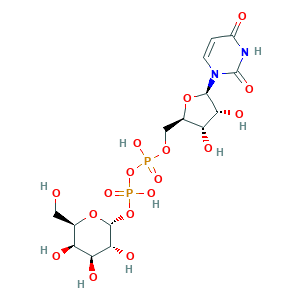
- Formula
- C15H24N2O17P2
- Canonical SMILES
- C1=CN(C(=O)NC1=O)C2C(C(C(O2)COP(=O)(O)OP(=O)(O)OC3C(C(C(C(O3)CO)O)O)O)O)O
- InChI
- 1S/C15H24N2O17P2/c18-3-5-8(20)10(22)12(24)14(32-5)33-36(28,29)34-35(26,27)30-4-6-9(21)11(23)13(31-6)17-2-1-7(19)16-15(17)25/h1-2,5-6,8-14,18,20-24H,3-4H2,(H,26,27)(H,28,29)(H,16,19,25)/t5-,6-,8+,9-,10+,11-,12-,13-,14-/m1/s1
- InChIKey
- HSCJRCZFDFQWRP-ABVWGUQPSA-N
|