| Synonyms |
Aminoglutethimide (AG); Aminoglutethimide (USP/INN); Aminoglutethimide [INN:BAN]; Aminoglutethimidum; Aminoglutethimidum [INN-Latin]; Aminoglutetimida; Aminoglutetimida [INN-Spanish]; Cytadren; Cytadren (TN); Dl-Aminoglutethimide; Elipten; Glutethimide, para-amino; Orimeten; P-Aminoglutethimide; (+-)-3-(p-Aminophenyl)-3-ethyl-2,6-piperidinedione; (+/-)-p-AMINOGLUTETHIMIDE; (inverted question mark)-p-Aminoglutethimide; 2-(p-Aminophenyl)-2-ethylglutarimide; 3-(4-Aminophenyl)-3-ethyl-2,6-piperidindion; 3-(4-Aminophenyl)-3-ethyl-2,6-piperidinedione; 3-(4-aminophenyl)-3-ethylpiperidine-2,6-dione; 3-(p-Aminophenyl)-3-ethylpiperidine-2,6-dione; 3-Ethyl-3-(p-aminophenyl)-2,6-dioxopiperidine; A 9657; AG-1; Alpha-(p-Aminophenyl)-alpha-ethylglutarimide; Ba 16038; Ba-16038; C 16038-BA; Ciba Vision Brand of Aminoglutethimide; Novartis Brand of Aminoglutethimide
|
| Cross-matching ID |
- PubChem CID
- 2145
- PubChem SID
-
9819
; 459146
; 3206291
; 4656527
; 7847640
; 7978687
; 8149197
; 8151456
; 10321343
; 10506109
; 11336059
; 11361298
; 11362814
; 11365376
; 11367938
; 11371432
; 11373727
; 11376100
; 11407292
; 11462270
; 11466272
; 11467392
; 11483782
; 11485983
; 11487909
; 11490112
; 11491959
; 11493854
; 12012594
; 15068844
; 17404687
; 24278142
; 25623233
; 26611601
; 26679683
; 26747446
; 26747447
; 26747448
; 29221324
; 46500462
; 46506066
; 46510177
; 47216836
; 47291191
; 47291192
; 47736545
; 47810808
; 48110505
; 48259296
; 48334561
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0M6DO
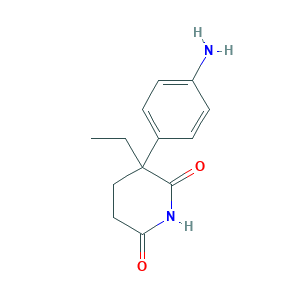
- Formula
- C13H16N2O2
- Canonical SMILES
- CCC1(CCC(=O)NC1=O)C2=CC=C(C=C2)N
- InChI
- 1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17)
- InChIKey
- ROBVIMPUHSLWNV-UHFFFAOYSA-N
|