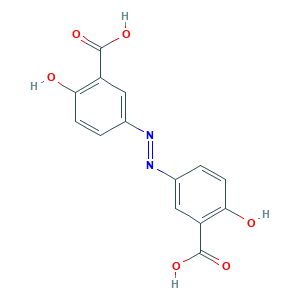
| Synonyms |
Olsalazine; Azodisal; Dipentium; Mirtazanine; Olsalazina; Olsalazina [Spanish]; Olsalazine (INN); Olsalazine [INN:BAN]; Olsalazinum; Olsalazinum [Latin]; Salicylic acid, 5,5'-azodi-; ULS5I8J03O; mordant yellow 5; (E)-5,5'-(diazene-1,2-diyl)bis(2-hydroxybenzoic acid); 15722-48-2; 3,3'-Azobis(6-hydroxybenzoic acid); 3,3'-diazene-1,2-diylbis(6-hydroxybenzoic acid); 5,5'-Azobis(salicylic acid); 5,5'-azodisalicylic acid; Benzoic acid, 3,3'-azobis(6-hydroxy-; C.I. Mordant Yellow 5; C14H10N2O6; CHEBI:7770; Rasal; UNII-ULS5I8J03O
|
| Cross-matching ID |
- PubChem CID
- 22419
- ChEBI ID
-
- CAS Number
-
- Formula
- C14H10N2O6
- Canonical SMILES
- C1=CC(=C(C=C1N=NC2=CC(=C(C=C2)O)C(=O)O)C(=O)O)O
- InChI
- 1S/C14H10N2O6/c17-11-3-1-7(5-9(11)13(19)20)15-16-8-2-4-12(18)10(6-8)14(21)22/h1-6,17-18H,(H,19,20)(H,21,22)
- InChIKey
- QQBDLJCYGRGAKP-UHFFFAOYSA-N
|