| Synonyms |
Methylated phytanic acid; MolPort-003-959-253; PHYTANIC ACID; Phytanate; Phytanoate; Phytanoic acid; RLCKHJSFHOZMDR-UHFFFAOYSA-N; SCHEMBL7888; 14721-66-5; 1986AH; 3,11,15-Tetramethylhexadecanoic acid; 3,7,11,15-Tetramethyl-hexadecansaeure; 3,7,11,15-Tetramethylhexadecanoic acid; 3,7,11,15-tetramethyl-hexadecanoic acid; 3,7,11,15-tetramethylhexadecoanoate; AC1L1BZT; AKOS028114496; BDBM119875; CHEBI:16285; CTK6A7614; GTPL2813; HMS3649L09; Hexadecanoic acid, 3,7,11,15-tetramethyl-; MFCD00133772; NSC 108698; NSC-108698; NSC108698
|
| Cross-matching ID |
- PubChem CID
- 26840
- PubChem SID
-
4760
; 408060
; 5028492
; 8143449
; 8169407
; 14752296
; 24898502
; 34669433
; 50270998
; 53787377
; 57310327
; 85088771
; 104298406
; 125334886
; 125817226
; 125817276
; 126523582
; 127271088
; 127271089
; 127271090
; 127271091
; 129144951
; 134990279
; 135651546
; 137005425
; 142113091
; 162093649
; 163241158
; 184585448
; 220230669
; 224034687
; 226399192
; 250133392
; 252457695
; 252468936
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05JXE
- Formula
- C20H40O2
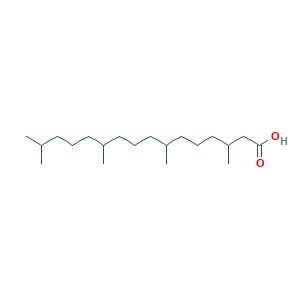
- Canonical SMILES
- CC(C)CCCC(C)CCCC(C)CCCC(C)CC(=O)O
- InChI
- 1S/C20H40O2/c1-16(2)9-6-10-17(3)11-7-12-18(4)13-8-14-19(5)15-20(21)22/h16-19H,6-15H2,1-5H3,(H,21,22)
- InChIKey
- RLCKHJSFHOZMDR-UHFFFAOYSA-N
|