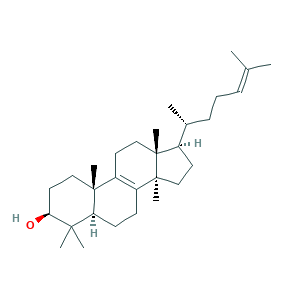
| Synonyms |
Botalan base; Botalan base 138; CAHGCLMLTWQZNJ-BQNIITSRSA-N; Lanosta-8,24-dien-3-beta-ol; Lanosta-8,24-dien-3-ol; Lanosta-8,24-dien-3-ol, (3beta)-; Lanosta-8,24-dien-3.beta.-ol; Lanosta-8,24-dien-3beta-ol; Lanosta-8,24-dienol; Lanostadien-3-beta-ol; Lanosterin; Lanster; lanosterol; (3beta)-lanosta-8,24-dien-3-ol; 1J05Z83K3M; 4,4',14alpha-Trimethyl-5alpha-cholesta-8,24-dien-3beta-ol; 79-63-0; CHEBI:16521; EINECS 201-214-9; Lanosta-8,24-dien-3-ol, (3.beta.)-; MFCD00021108; NSC 60677; NSC60677; UNII-1J05Z83K3M
|
| Cross-matching ID |
- PubChem CID
- 246983
- PubChem SID
-
2905
; 4861
; 108662
; 4266378
; 7888607
; 8138329
; 8143526
; 9395181
; 12158930
; 15302777
; 24896283
; 24896417
; 25651433
; 26757817
; 30045540
; 57309786
; 57400449
; 79349088
; 87565551
; 87623957
; 92298600
; 103514947
; 104491139
; 126523909
; 127287164
; 127287165
; 127287166
; 134368260
; 135651499
; 137237308
; 140346043
; 162094640
; 163667483
; 164811548
; 166211000
; 179293068
; 198956768
; 226512170
; 241225868
; 249929901
; 252402925
; 252450022
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0P4PQ
- Formula
- C30H50O
- Canonical SMILES
- CC(CCC=C(C)C)C1CCC2(C1(CCC3=C2CCC4C3(CCC(C4(C)C)O)C)C)C
- InChI
- 1S/C30H50O/c1-20(2)10-9-11-21(3)22-14-18-30(8)24-12-13-25-27(4,5)26(31)16-17-28(25,6)23(24)15-19-29(22,30)7/h10,21-22,25-26,31H,9,11-19H2,1-8H3/t21-,22-,25+,26+,28-,29-,30+/m1/s1
- InChIKey
- CAHGCLMLTWQZNJ-BQNIITSRSA-N
|