Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR2679) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Ceftazidine
|
|||||
| Synonyms |
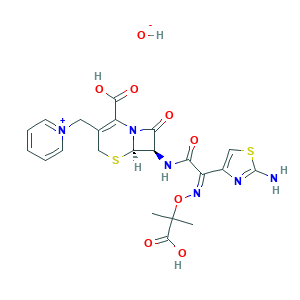
Ceftazidine [INN]; Ceftazidine [USAN]; Ceftazidine [IBAN]; (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(2-carboxypropan-2-yloxyimino)acetyl]amino]-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;hydroxide
|
|||||
| Indication | Meningitis [ICD11: 1A62] | Phase 4 | [1] | |||
| Structure |
 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 564.6 | Topological Polar Surface Area | 243 | ||
| Heavy Atom Count | 38 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 13 | |||
| Cross-matching ID |
|
|||||
| The Predicted Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Predicted Drug Metabolites (PDM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

