| General Information of Drug (ID:
DR3110) |
| Drug Name |
CC-122
|
| Synonyms |
cc-122; 1015474-32-4; Avadomide; 3-(5-Amino-2-methyl-4-oxoquinazolin-3(4H)-yl)piperidine-2,6-dione; CC122; CC 122; 3-(5-amino-2-methyl-4-oxo-4H-quinazolin-3-yl)-piperidine-2,6-dione; 2,6-Piperidinedione, 3-(5-amino-2-methyl-4-oxo-3(4H)-quinazolinyl)-;2,6-Piperidinedione, 3-(5-amino-2-methyl-4-oxo-3(4H)-quinazolinyl)-; Avadomide [USAN]; Avadomide(CC-122); Avadomide (USAN/INN); SCHEMBL282749; US9694015, Compound A; CHEMBL3989934; BDBM76986; RSNPAKAFCAAMBH-UHFFFAOYSA-N; EX-A1191; BCP15938; s7892; AKOS025399378; SB18829; CS-5995
|
| Indication |
Brain cancer
[ICD11: 2A00]
|
Phase 1
|
[1]
|
|
Chronic lymphocytic leukaemia
[ICD11:
ICD11: 2A82]
|
Phase 1
|
[2]
|
|
Diffuse large B-cell lymphoma
[ICD11:
ICD11: 2A81]
|
Phase 1
|
[3]
|
|
Hepatocellular carcinoma
[ICD11:
ICD11: 2C12]
|
Phase 1
|
[4]
|
|
Lymphoma
[ICD11:
ICD11: 2A80-2A86]
|
Phase 1
|
[5]
|
|
Multiple myeloma
[ICD11:
ICD11: 2A83]
|
Phase 1
|
[6]
|
|
Solid tumour/cancer
[ICD11:
ICD11: 2A00-2F9Z]
|
Phase 1
|
[7]
|
| Structure |
|
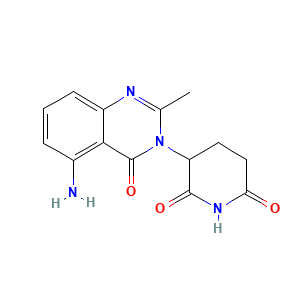 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
286.29 |
Topological Polar Surface Area |
105 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
1 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 24967599
- CAS Number
-
- TTD Drug ID
- D03QZJ
- Formula
- C14H14N4O3
- Canonical SMILES
- CC1=NC2=CC=CC(=C2C(=O)N1C3CCC(=O)NC3=O)N
- InChI
- InChI=1S/C14H14N4O3/c1-7-16-9-4-2-3-8(15)12(9)14(21)18(7)10-5-6-11(19)17-13(10)20/h2-4,10H,5-6,15H2,1H3,(H,17,19,20)
- InChIKey
- RSNPAKAFCAAMBH-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


