| Synonyms |
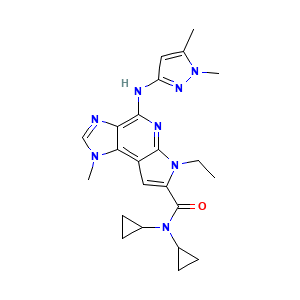
BMS-911543; 1271022-90-2; BMS 911543; BMS911543; UNII-7N03P021J8; 7N03P021J8; N,N-Dicyclopropyl-4-[(1,5-Dimethyl-1h-Pyrazol-3-Yl)amino]-6-Ethyl-1-Methyl-1,6-Dihydroimidazo[4,5-D]pyrrolo[2,3-B]pyridine-7-Carboxamide; N,N-dicyclopropyl-4-((1,5-dimethyl-1H-pyrazol-3-yl)amino)-6-ethyl-1-methyl-1,6-dihydroimidazo[4,5-d]pyrrolo[2,3-b]pyridine-7-carboxamide; N,N-dicyclopropyl-4-((1,5-dimethyl-1H-pyrazol-3-yl)amino)-6-ethyl-1-methyl-1,6-dihydroimidazo(4,5-d)pyrrolo(2,3b)pyridine-7-carboxamide; JCINBYQJBYJGDM-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 50922691
- CAS Number
-
- TTD Drug ID
- D0DK8X
- Formula
- C23H28N8O
- Canonical SMILES
- CCN1C(=CC2=C3C(=C(N=C21)NC4=NN(C(=C4)C)C)N=CN3C)C(=O)N(C5CC5)C6CC6
- InChI
- InChI=1S/C23H28N8O/c1-5-30-17(23(32)31(14-6-7-14)15-8-9-15)11-16-20-19(24-12-28(20)3)21(26-22(16)30)25-18-10-13(2)29(4)27-18/h10-12,14-15H,5-9H2,1-4H3,(H,25,26,27)
- InChIKey
- JCINBYQJBYJGDM-UHFFFAOYSA-N
|