| Synonyms |
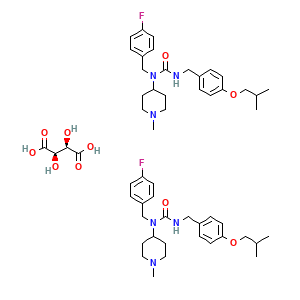
Pimavanserin tartrate; UNII-NA83F1SJSR; 706782-28-7; ACP 103; ACP-103; 706782-28-7 (tartrate); NA83F1SJSR; Pimavanserin tartrate [USAN]; Bis(1-(4-Fluorobenzyl)-1-(1-methylpiperidin-4-yl)-3-(4-(2-methylpropoxy)benzyl)urea) (2R,3R)-2,3-dihydroxybutanedioate; Pimavanserin tartrate (USAN); 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-(1-methylpiperidin-4-yl)urea, ((2R,3R)-2,3-dihydroxysuccinate) (2:1); Nuplazide (TN); pimavanserin hemitartrate; DTXSID50220958; CHEBI:133014; HMS3886L06; HY-14557A; Pimavanserin Dihydroxysuccinate(2:1); AKOS027327334; CCG-270608; CS-7954; 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-(1-methylpiperidin-4-yl)urea (2R,3R)-2,3-dihydroxysuccinate; AC-29901; AS-56699; N-(4-Fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1); Urea, N-((4-fluorophenyl)methyl)-N-(1-methyl-4-piperidinyl)-N'-((4-(2-methylpropoxy)phenyl)methyl)-, (2R,3R)-2,3-dihydroxybutanedioate (2:1); D08969; Q27284759; bis(4-{[(4-fluorophenyl)methyl]({[4-(2-methylpropoxy)phenyl]methyl}carbamoyl)amino}-1-methylpiperidin-1-ium) (2R,3R)-2,3-dihydroxybutanedioate; bis{N-[(4-fluorophenyl)methyl]-N-(1-methylpiperidin-4-yl)-N'-{[4-(2-methylpropoxy)phenyl]methyl}urea} (2R,3R)-2,3-dihydroxybutanedioate
|
| Cross-matching ID |
- PubChem CID
- 11672491
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- DDI6B0
- Formula
- C54H74F2N6O10
- Canonical SMILES
- CC(C)COC1=CC=C(C=C1)CNC(=O)N(CC2=CC=C(C=C2)F)C3CCN(CC3)C.CC(C)COC1=CC=C(C=C1)CNC(=O)N(CC2=CC=C(C=C2)F)C3CCN(CC3)C.[C@@H]([C@H](C(=O)O)O)(C(=O)O)O
- InChI
- InChI=1S/2C25H34FN3O2.C4H6O6/c2*1-19(2)18-31-24-10-6-20(7-11-24)16-27-25(30)29(23-12-14-28(3)15-13-23)17-21-4-8-22(26)9-5-21;5-1(3(7)8)2(6)4(9)10/h2*4-11,19,23H,12-18H2,1-3H3,(H,27,30);1-2,5-6H,(H,7,8)(H,9,10)/t;;1-,2-/m..1/s1
- InChIKey
- RGSULKHNAKTFIZ-CEAXSRTFSA-N
|