| Synonyms |
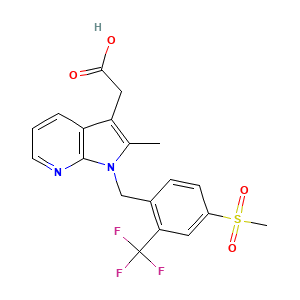
872365-14-5; NVP-QAW039; QAW039; UNII-2PEX5N7DQ4; 2PEX5N7DQ4; 2-[2-methyl-1-[[4-methylsulfonyl-2-(trifluoromethyl)phenyl]methyl]pyrrolo[2,3-b]pyridin-3-yl]acetic acid; 2-(2-methyl-1-(4-(methylsulfonyl)-2-(trifluoromethyl)benzyl)-1H-pyrrolo[2,3-b]pyridin-3-yl)acetic acid; 2-Methyl-1-[[4-(methylsulfonyl)-2-(trifluoromethyl)phenyl]methyl]-1H-pyrrolo[2,3-b]pyridine-3-acetic acid; Fevipiprant [INN]; Fevipiprant [USAN:INN]; QAW 039; Fevipiprant (JAN/USAN/INN); GTPL8995; SCHEMBL1940595; CHEMBL3137332; QAW-039; HMS3743E19; BCP25015; EX-A2495; BDBM50233520; ZINC43101772; AB85348; CS-5956; DB12011; Fevipiprant; NVP-QAW039; QAW039; SB16897; (1-(4-((Methane)sulfonyl)-2-trifluoromethylbenzyl)-2-methyl-1H-pyrrolo(2,3-b)pyridin-3-yl)acetic acid; 2-(1-((4-Methanesulfonyl-2-(trifluoromethyl)phenyl)methyl(-2-methyl-1H-pyrrolo(2,3-b)pyridin-3-yl)acetic acid; AC-31956; AS-74870; HY-16768; DS-022511; FT-0774596; D10631; Q27077292; 1-(4-methanesulfonylbenzyl)-2-methyl-1H-pyrrolo(2,3-b)pyridin-3-yl)acetic acid; (2-methyl-1-{[4-(methylsulfonyl)-2-(trifluoromethyl)phenyl]methyl}-1H-pyrrolo[2,3-b]pyridin-3-yl)acetic acid; [1-(4-Methanesulfonyl-2-trifluoromethyl-benzyl)-2-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl]-acetic acid; [2-METHYL-1-[4-(METHYLSULFONYL)-2-(TRIFLUOROMETHYL)BENZYL]-1H-PYRROLO[2,3-B]PYRIDIN-3-YL]ACETIC ACID; 2-(1-{[4-methanesulfonyl-2-(trifluoromethyl)phenyl]methyl}-2-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl)acetic acid; 2-[2-methyl-1-[[4-methylsulfonyl-2-(trifluoromethyl)phenyl]methyl]pyrrolo[5,4-b]pyridin-3-yl]acetic acid; FSY
|
| Cross-matching ID |
- PubChem CID
- 23582412
- CAS Number
-
- TTD Drug ID
- DH7F9S
- Formula
- C19H17F3N2O4S
- Canonical SMILES
- CC1=C(C2=C(N1CC3=C(C=C(C=C3)S(=O)(=O)C)C(F)(F)F)N=CC=C2)CC(=O)O
- InChI
- InChI=1S/C19H17F3N2O4S/c1-11-15(9-17(25)26)14-4-3-7-23-18(14)24(11)10-12-5-6-13(29(2,27)28)8-16(12)19(20,21)22/h3-8H,9-10H2,1-2H3,(H,25,26)
- InChIKey
- GFPPXZDRVCSVNR-UHFFFAOYSA-N
|