Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR3496) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Almonertinib
|
|||||
| Synonyms |
HS-10296; Ameile; 1899921-05-1; Egfr T790M inhibitor HS-10296; N-(5-((4-(1-Cyclopropyl-1H-indol-3-yl)pyrimidin-2-yl)amino)-2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxyphenyl)acrylamide; UNII-T4RS462G19; SCHEMBL17683063; GTPL11136; T4RS462G19; EX-A3721; HS 10296 [WHO-DD]; s8817; HS10296; example 26 [WO2016054987A1]; HY-112823; CS-0066162; 2-Propenamide, N-(5-((4-(1-cyclopropyl-1H-indol-3-yl)-2-pyrimidinyl)amino)-2-((2-(dimethylamino)ethyl)methylamino)-4-methoxyphenyl)-; C1(CC1)N1C=C(C2=CC=CC=C12)C1=NC(=NC=C1)NC=1C(=CC(=C(C=1)NC(C=C)=O)N(C)CCN(C)C)OC; N-[5-[[4-(1-cyclopropylindol-3-yl)pyrimidin-2-yl]amino]-2-[2-(dimethylamino)ethyl-methylamino]-4-methoxyphenyl]prop-2-enamide
|
|||||
| Indication | Lung cancer [ICD11: 2C25] | Phase 3 | [1] | |||
| Structure |
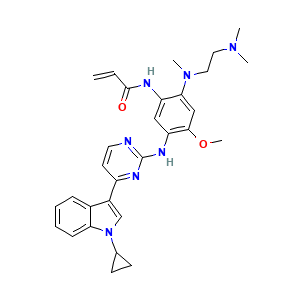 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 525.6 | Topological Polar Surface Area | 87.6 | ||
| Heavy Atom Count | 39 | Rotatable Bond Count | 11 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | ClinicalTrials.gov (NCT04870190) Almonertinib Versus Osimertinib in the Patients With EGFR Mutations in Advanced NSCLC With Brain Metastases (ATTACK). U.S. National Institutes of Health. | |||||
| 2 | Itraconazole and rifampicin, as CYP3A modulators but not P-gp modulators, affect the pharmacokinetics of almonertinib and active metabolite HAS-719 in healthy volunteers | |||||
| 3 | Absorption, metabolism, excretion, and safety of [(14)C]almonertinib in healthy Chinese subjects | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

