Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR5010) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Clazosentan
|
|||||
| Synonyms |
Clazosentan; 180384-56-9; Ro-61-1790; VML-588; UNII-3DRR0X4728; AXV-343434; CHEMBL109648; 3DRR0X4728; Clazosentan [INN]; VML 588; AXV 034; AXV-034343; Ro 61-1790; AXV-034; AXV 034343; AC1O5FK3; SCHEMBL1652657; CTK4D7597; DTXSID60170955; ZINC3939238; BDBM50066370; AKOS032946377; SB18855; N-[6-(2-HYDROXYETHOXY)-5-(2-METHOXYPHENOXY)-2-[2-(2H-TETRAZOL-5-YL)-4-PYRIDINYL]-4-PYRIMIDINYL]-5-METHYL-2-PYRIDINESULFONAMIDE; DB-065318; FT-0765561
|
|||||
| Indication | Cerebral vasospasm [ICD11: BA85] | Phase 3 | [1] | |||
| Vasospasm [ICD11: ICD11: 9B74] | Discontinued in Phase 3 | [2] | ||||
| Structure |
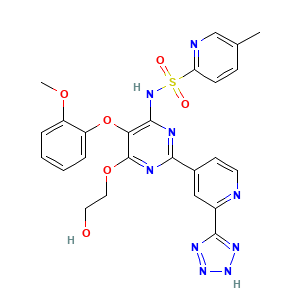 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 577.6 | Topological Polar Surface Area | 209 | ||
| Heavy Atom Count | 41 | Rotatable Bond Count | 11 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 14 | |||
| Cross-matching ID |
|
|||||
| The Predicted Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Predicted Drug Metabolites (PDM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

