| General Information of Drug (ID:
DR5143) |
| Drug Name |
Cephaloglycin
|
| Synonyms |
CEG; Cefaloglicina; Cefaloglycin; Cefaloglycine; Cefaloglycinum; Cephaloglycine; Kafocin; Kefglycin; Cephaloglycinanhdyous; Cephaloglycin anhydrous; Cephaoglycin acid; Lilly 39435; Cefaloglicina [INN-Spanish]; Cefaloglycin (JAN); Cefaloglycine [INN-French]; Cefaloglycinum [INN-Latin]; Cephaloglycin (anhydrous); D-Cephaloglycine; D-(-)-Cephaloglycin; (6R,7R)-3-(acetoxymethyl)-7-{[(2R)-2-amino-2-phenylacetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-(acetyloxymethyl)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-[(acetyloxy)methyl]-7-{[(2R)-2-amino-2-phenylacetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 3-((Acetyloxy)methyl)-7-((aminophenylacetyl)amino)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; 3-acetoxymethyl-7beta-[(2R)-2-amino-2-phenylacetamido]-3,4-didehydrocepham-4-carboxylic acid; 7-(2-Amino-2-phenylacetamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid acetate (ester); 7-(2-Amino-2-phenylacetamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)octane-2-carboxylic acid, acetate inner salt; 7-(2-D-alpha-Aminophenylacetamido)cephalosporanic acid; 7-(D-2-Amino-2-phenylacetamido)-3-acetoxymethyl-delta(sup 3)-cephem-4-carboxylic acid; 7-(D-2-Amino-2-phenylacetamido)-3-acetoxymethyl-delta(sup3)-cephem-4-carboxylic acid; 7-(D-alpha-Aminophenyl-acetamido)cephalosporanic acid
|
| Indication |
Bacterial infection
[ICD11: 1A00-1C4Z]
|
Approved
|
[1]
|
| Structure |
|
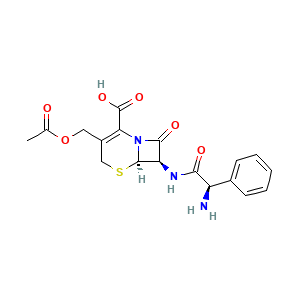 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
405.4 |
Topological Polar Surface Area |
164 |
| Heavy Atom Count |
28 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 19150
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07WZH
- Formula
- C18H19N3O6S
- Canonical SMILES
- CC(=O)OCC1=C(N2[C@@H]([C@@H](C2=O)NC(=O)[C@@H](C3=CC=CC=C3)N)SC1)C(=O)O
- InChI
- InChI=1S/C18H19N3O6S/c1-9(22)27-7-11-8-28-17-13(16(24)21(17)14(11)18(25)26)20-15(23)12(19)10-5-3-2-4-6-10/h2-6,12-13,17H,7-8,19H2,1H3,(H,20,23)(H,25,26)/t12-,13-,17-/m1/s1
- InChIKey
- FUBBGQLTSCSAON-PBFPGSCMSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


