| Synonyms |
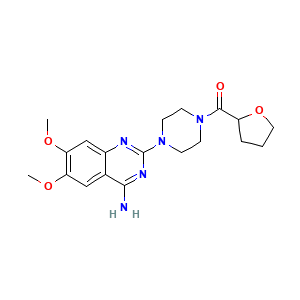
Blavin; Flumarc; Fosfomic; Hytracin; Hytrin; Terazosabb; Terazosina; Terazosine; Terazosinum; Vasomet; Trazosin HCl; A 45975; Abbott 45975; A-45975; Hytrin (TN); Terazosabb (TN); Terazosin (INN); Terazosin [INN:BAN]; Terazosina [INN-Spanish]; Terazosine [INN-French]; Terazosinum [INN-Latin]; [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](tetrahydrofuran-2-yl)methanone; [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(oxolan-2-yl)methanone; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-((tetrahydro-2-furanyl)carbonyl)piperazine; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(tetrahydro-2-furoyl)piperazine; 6,7-bis(methyloxy)-2-[4-(tetrahydrofuran-2-ylcarbonyl)piperazin-1-yl]quinazolin-4-amine; 6,7-dimethoxy-2-[4-(tetrahydrofuran-2-ylcarbonyl)piperazin-1-yl]quinazolin-4-amine
|
| Cross-matching ID |
- PubChem CID
- 5401
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0P9RF
- Formula
- C19H25N5O4
- Canonical SMILES
- COC1=C(C=C2C(=C1)C(=NC(=N2)N3CCN(CC3)C(=O)C4CCCO4)N)OC
- InChI
- InChI=1S/C19H25N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h10-11,14H,3-9H2,1-2H3,(H2,20,21,22)
- InChIKey
- VCKUSRYTPJJLNI-UHFFFAOYSA-N
|