Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR5367) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Fedratinib
|
|||||
| Synonyms | TG101348 | |||||
| Indication | Chronic myelogenous leukaemia [ICD11: 2A20] | Approved | [1] | |||
| Structure |
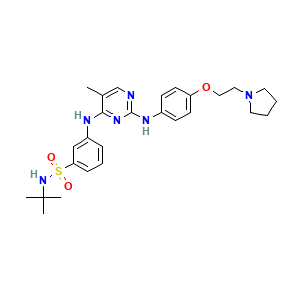 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 524.7 | Topological Polar Surface Area | 117 | ||
| Heavy Atom Count | 37 | Rotatable Bond Count | 11 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 9 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019 | |||||
| 2 | Effects of strong and moderate CYP3A4 inducers on the pharmacokinetics of fedratinib in healthy adult participants | |||||
| 3 | In vivo Pharmacokinetic Drug-Drug Interaction Studies Between Fedratinib and Antifungal Agents Based on a Newly Developed and Validated UPLC/MS-MS Method Front Pharmacol. 2021 Feb 5;11:626897. doi: 10.3389/fphar.2020.626897. | |||||
| 4 | Effect of fluconazole on the pharmacokinetics of a single dose of fedratinib in healthy adults. Cancer Chemother Pharmacol. 2022 Oct;90(4):325-334. doi: 10.1007/s00280-022-04464-w. | |||||
| 5 | A Comprehensive Overview of Globally Approved JAK Inhibitors | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

