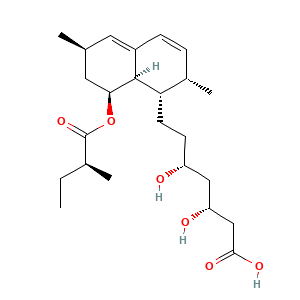
| Synonyms |
Lovastatin acid; Mevinolinic acid; Monacolinic K acid; MSD 803 acid; MSD 803 free acid; UNII-5CLV35Y90C; MK 819; L-154819; L 154819; 5CLV35Y90C; CHEBI:82985; (3R,5R)-7-((1R,2R,6S,8R,8AS)-2,6-DIMETHYL-8-{[(2R)-2-METHYLBUTANOYL]OXY}-1,2,6,7,8,8A-HEXAHYDRONAPHTHALEN-1-YL)-3,5-DIHYDROXYHEPTANOIC ACID; (3R,5R)-7-[(1S,2S,6R,8S,8aR)-2,6-dimethyl-8-{[(2S)-2-methylbutanoyl]oxy}-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoic acid
|
| Cross-matching ID |
- PubChem CID
- 64727
- ChEBI ID
-
- CAS Number
-
- Formula
- C24H38O6
- Canonical SMILES
- CCC(C)C(=O)OC1CC(C=C2C1C(C(C=C2)C)CCC(CC(CC(=O)O)O)O)C
- InChI
- InChI=1S/C24H38O6/c1-5-15(3)24(29)30-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-18(25)12-19(26)13-22(27)28/h6-7,10,14-16,18-21,23,25-26H,5,8-9,11-13H2,1-4H3,(H,27,28)/t14-,15-,16-,18+,19+,20-,21-,23-/m0/s1
- InChIKey
- QLJODMDSTUBWDW-BXMDZJJMSA-N
|