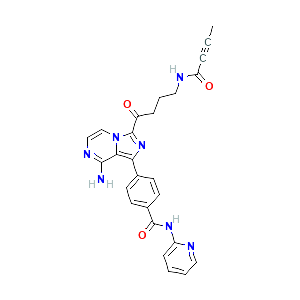
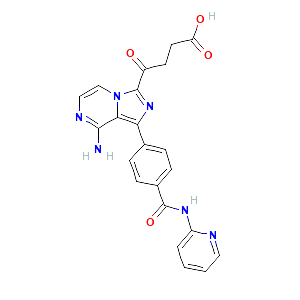
| References |
| 1 |
Bioavailability, Biotransformation, and Excretion of the Covalent Bruton Tyrosine Kinase Inhibitor Acalabrutinib in Rats, Dogs, and Humans Drug Metab Dispos. 2019 Feb;47(2):145-154. doi: 10.1124/dmd.118.084459.
|
| 2 |
Identification and Characterization of ACP-5862, the Major Circulating Active Metabolite of Acalabrutinib: Both Are Potent and Selective Covalent Bruton Tyrosine Kinase Inhibitors?. J Pharmacol Exp Ther. 2023 Jan;384(1):173-186. doi: 10.1124/jpet.122.001116.
|
| 3 |
Bioequivalence and Relative Bioavailability Studies to Assess a New Acalabrutinib Formulation That Enables Coadministration With Proton-Pump Inhibitors. Clin Pharmacol Drug Dev. 2022 Nov;11(11):1294-1307. doi: 10.1002/cpdd.1153.
|