Details of the Metabolic Reaction (MR)
| General Information of This Metabolic Reaction (MR) (ID: MR004104) | ||||||
|---|---|---|---|---|---|---|
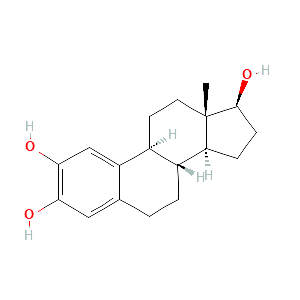
| Formula |
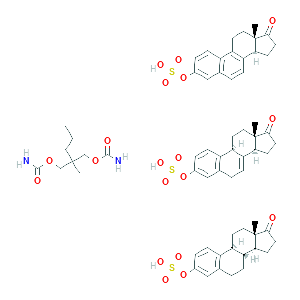 Hydrolyzationn
 |
|||||
| Reactant | Conjugated estrogens | Product | 2-hydroxyestradiol | |||
| Reactant Info | Product Info | |||||
| Metabolic Type | Oxidation - Hydrolyzationn | |||||
| Other MR(s) Related to The Reactant of This MR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Other MR(s) That Metabolize The Reactant of This MR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other MR(s) Related to The Product of This MR | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Other MR(s) That Produce The Product of This MR | |||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

