Detail Information of Xenobiotics
| General Information of Xenobiotics (ID: XEO00583) | ||||||
|---|---|---|---|---|---|---|
| Xenobiotics Name |
NS-398
|
|||||
| Xenobiotics Type |
Pharmaceutical Agent(s)
|
|||||
| Classification |
Drug in Phase 1 Clinical Trial
|
|||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=4553"></iframe>
|
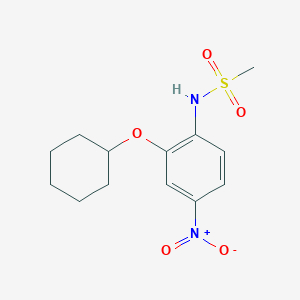 |
||||
| 3D MOL | 2D MOL | |||||
| PubChem CID | ||||||
| DME(s) Modulated by This Xenobiotics | ||||||
| DME(s) Inhibited by This Xenobiotics | ||||||
| Aromatase (CYP19A1) | DME Info | Homo sapiens | [1], [2] | |||
| Prostaglandin G/H synthase 1 (COX-1) | DME Info | Homo sapiens | [3] | |||
| Prostaglandin G/H synthase 2 (COX-2) | DME Info | Homo sapiens | [4] | |||
| DME(s) Induced by This Xenobiotics | ||||||
| Arachidonate 15-lipoxygenase (ALOX15) | DME Info | Homo sapiens | [5] | |||
| Cellular glutathione peroxidase (GPX1) | DME Info | Homo sapiens | [5] | |||
| Xenobiotics-DME Activity Data | ||||||
| Xenobiotics-DME Activity Data | Aromatase (CYP19A1) | DME Info | IC50 = 0.68 microM | [1], [2] | ||
| Prostaglandin G/H synthase 1 (COX-1) | DME Info | IC50 = 220 microM | [3] | |||
| Prostaglandin G/H synthase 2 (COX-2) | DME Info | IC50 = 0.001 microM | [4] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

