Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0021) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Methoxybufotenin
|
|||||
| Synonyms |
Bufotenine, O-methyl-; MeODMT; 5-Methoxy-N,N-Dimethyltryptamine; Methoxydimethyltryptamines; Methylbufotenine; N,N-Dimethyl-5-methoxytryptamine; O-Methylbufotenine; 1019-45-0; 1H-Indole-3-ethanamine, 5-methoxy-N,N-dimethyl-; 2-(5-Methoxy-1H-indol-3-yl)-N,N-dimethylethanamine; 3-(2-Dimethylaminoethyl)-5-methoxyindole; 5-MeO-DMT; 5-Methoxy-N,N-dimethyl-1H-indole-3-ethanamine; 5-Methoxy-N,N-dimethyltryptamine; 5-Methoxydimethyltryptamine; 5-OMe-DMT; CT 4334; INDOLE, 3-(2-(N,N-DIMETHYLAMINO)ETHYL)-5-METHOXY-; NSC 88624; UNII-X0MKX3GWU9
|
|||||
| Indication | Discovery agent | Investigative | [1] | |||
| Structure |
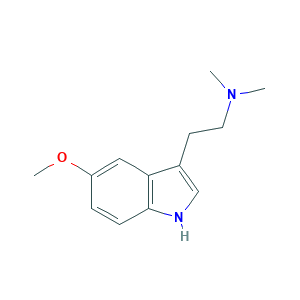 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 218.29 | Topological Polar Surface Area | 28.3 | ||
| Heavy Atom Count | 16 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

