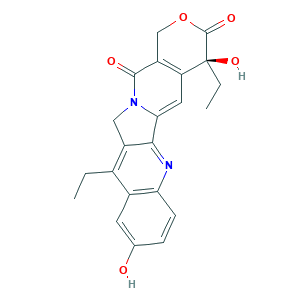
| Synonyms |
Captothecin, 7-ethyl-10-hydroxy-; SN 38 lactone; (+)-7-ETHYL-10-HYDROXYCAMPTOTHECIN; (S)-4,11-Diethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; 0H43101T0J; 10-Hydroxy-7-ethylcamptothecin; 7-Ethyl-10-hydroxy camptothecin; 7-Ethyl-10-hydroxy-20(S)-camptothecin; 7-Ethyl-10-hydroxy-camptothecin; 7-Ethyl-10-hydroxycamptothecin; 7-Ethyl-10-hydroxycamptothecine; 86639-52-3; CHEBI:8988; NSC673596; SN 38; SN38; UNII-0H43101T0J
|
| Cross-matching ID |
- PubChem CID
- 104842
- PubChem SID
-
13355
; 515301
; 622834
; 10233636
; 11430409
; 12012598
; 14805294
; 14805295
; 44434437
; 49835447
; 50028359
; 50112971
; 56311337
; 56311445
; 56311571
; 56312630
; 57337873
; 71831605
; 80486942
; 87559919
; 89360249
; 92712502
; 103220117
; 103996165
; 104373061
; 126588659
; 126625532
; 126656661
; 126666539
; 127855413
; 134340412
; 134340540
; 135062353
; 135693781
; 135710073
; 136377612
; 136897516
; 136911131
; 137138435
; 142309355
; 144115510
; 152059852
; 152164367
; 152200177
; 152245349
; 162178284
; 162223790
; 163367112
; 164178264
; 164194982
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0B6YB
- Formula
- C22H20N2O5
- Canonical SMILES
- CCC1=C2CN3C(=CC4=C(C3=O)COC(=O)C4(CC)O)C2=NC5=C1C=C(C=C5)O
- InChI
- 1S/C22H20N2O5/c1-3-12-13-7-11(25)5-6-17(13)23-19-14(12)9-24-18(19)8-16-15(20(24)26)10-29-21(27)22(16,28)4-2/h5-8,25,28H,3-4,9-10H2,1-2H3/t22-/m0/s1
- InChIKey
- FJHBVJOVLFPMQE-QFIPXVFZSA-N
|