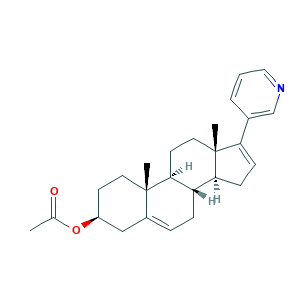
| Synonyms |
Abiraterone (acetate); Abiraterone acetate; Abiraterone; Abiraterone (CB-7598); CB 7598; CB-7598; CB7598; CB 7630; CB-7630; CB7630; CHEBI:68639; EM5OCB9YJ6; UNII-EM5OCB9YJ6; Yonsa; Zytiga; CHEBI:68642; CHEMBL254328; G819A456D0; MFCD00924100; UNII-G819A456D0; [(3S,8R,9S,10R,13S,14S)-10,13-dimethyl-17-pyridin-3-yl-2,3,4,7,8,9,11,12,14,15-decahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate; (3S,8R,9S,10R,13S,14S)-10,13-DIMETHYL-17-(PYRIDIN-3-YL)-2,3,4,7,8,9,10,11,12,13,14,15-DODECAHYDRO-1H-CYCLOPENTA[A]PHENANTHREN-3-YL ACETATE; 154229-18-2; 17-(3-Pyridyl)-5,16-androstadien-3beta-acetate; 17-(pyridin-3-yl)androsta-5,16-dien-3beta-yl acetate; (3S,8R,9S,10R,13S,14S)-10,13-dimethyl-17-(pyridin-3-yl)-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; (3beta)-17-(3-pyridinyl)-androsta-5,16-dien-3-ol; (3beta)-17-(pyridin-3-yl)androsta-5,16-dien-3-ol; 154229-19-3; 17-(3-Pyridyl)androsta-5,16-dien-3beta-ol
|
| Cross-matching ID |
- PubChem CID
- 9821849
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06CNP
- Formula
- C26H33NO2
- Canonical SMILES
- CC(=O)OC1CCC2(C3CCC4(C(C3CC=C2C1)CC=C4C5=CN=CC=C5)C)C
- InChI
- 1S/C26H33NO2/c1-17(28)29-20-10-12-25(2)19(15-20)6-7-21-23-9-8-22(18-5-4-14-27-16-18)26(23,3)13-11-24(21)25/h4-6,8,14,16,20-21,23-24H,7,9-13,15H2,1-3H3/t20-,21-,23-,24-,25-,26+/m0/s1
- InChIKey
- UVIQSJCZCSLXRZ-UBUQANBQSA-N
|