Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0073) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Alosetron hydrochloride
|
|||||
| Synonyms |
Alosetron (Hydrochloride); Alosetron hydrochloride [USAN]; C17H19ClN4O; DSSTox_CID_24208; DSSTox_GSID_44208; DSSTox_RID_80120; GR 68755; GR 68755X; GR 68755c; GR-68755C; HSDB 7055; UNII-2F5R1A46YW; alosetron monohydrochloride; 122852-69-1; 2,3,4,5-Tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-1H-pyrido[4,3-b]indol-1-one hydrochloride; 2F5R1A46YW; 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one hydrochloride; ALOSETRON HYDROCHLORIDE
|
|||||
| Indication | Irritable bowel syndrome [ICD11: DD91] | Approved | [1] | |||
| Structure |
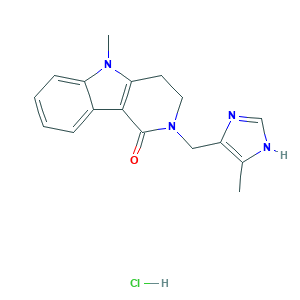 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 330.8 | Topological Polar Surface Area | 53.9 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 2 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Alosetron Hydrochloride was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | Optimizing outcomes with alosetron hydrochloride in severe diarrhea-predominant irritable bowel syndrome. Therap Adv Gastroenterol. 2010 May;3(3):165-72. | |||||
| 3 | Effect of alosetron on the pharmacokinetics of fluoxetine. J Clin Pharmacol. 2001 Apr;41(4):455-8. | |||||
| 4 | Effect of alosetron on the pharmacokinetics of alprazolam. J Clin Pharmacol. 2001 Apr;41(4):452-4. | |||||
| 5 | Characterization of the metabolites of alosetron in experimental animals and human | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

