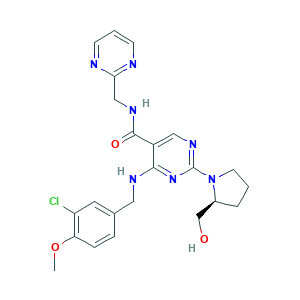
| Cross-matching ID |
- PubChem CID
- 9869929
- PubChem SID
-
14834924
; 15132057
; 17397370
; 24160918
; 45292193
; 50284293
; 57373612
; 125299302
; 126680642
; 135264773
; 136922931
; 137082779
; 137263447
; 143177981
; 160645587
; 162255031
; 164178031
; 164194088
; 164835991
; 165222624
; 165245576
; 170502146
; 174528935
; 175267514
; 175427065
; 178104020
; 185997047
; 189480340
; 198993783
; 210275731
; 210281392
; 223635467
; 223667975
; 223700581
; 223797060
; 226490661
; 241381281
; 244115505
; 249855750
; 250163172
; 250224291
; 252066590
; 252214915
; 252436466
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Y5JC
- Formula
- C23H26ClN7O3
- Canonical SMILES
- COC1=C(C=C(C=C1)CNC2=NC(=NC=C2C(=O)NCC3=NC=CC=N3)N4CCCC4CO)Cl
- InChI
- 1S/C23H26ClN7O3/c1-34-19-6-5-15(10-18(19)24)11-27-21-17(22(33)28-13-20-25-7-3-8-26-20)12-29-23(30-21)31-9-2-4-16(31)14-32/h3,5-8,10,12,16,32H,2,4,9,11,13-14H2,1H3,(H,28,33)(H,27,29,30)/t16-/m0/s1
- InChIKey
- WEAJZXNPAWBCOA-INIZCTEOSA-N
|