| Synonyms |
Boceprevir; Victrelis; EBP 520; SCH 503034; SCH-503034; (1R,2S,5S)-N-(4-amino-1-cyclobutyl-3,4-dioxobutan-2-yl)-3-[N-(tert-butylcarbamoyl)-3-methyl-L-valyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide; 3-{[(1R,2S,5S)-3-[(2S)-2-[(tert-butylcarbamoyl)amino]-3,3-dimethylbutanoyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-yl]formamido}-4-cyclobutyl-2-oxobutanamide; 394730-60-0; 89BT58KELH; CHEBI:68621; UNII-89BT58KELH
|
| Cross-matching ID |
- PubChem CID
- 10324367
- PubChem SID
-
15333889
; 22433429
; 22693607
; 41390184
; 46512784
; 57374555
; 57414843
; 79603721
; 92309409
; 96025559
; 103500897
; 109692987
; 134348397
; 135216213
; 137186019
; 137251857
; 139040316
; 152258143
; 160645839
; 160646982
; 162256796
; 163667336
; 164837112
; 175427026
; 175427127
; 176245990
; 180371765
; 198992422
; 223366205
; 223669397
; 226940314
; 245972903
; 249582863
; 251970943
; 252076908
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0V3YT
- Formula
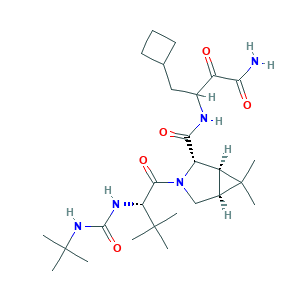
- C27H45N5O5
- Canonical SMILES
- CC1(C2C1C(N(C2)C(=O)C(C(C)(C)C)NC(=O)NC(C)(C)C)C(=O)NC(CC3CCC3)C(=O)C(=O)N)C
- InChI
- 1S/C27H45N5O5/c1-25(2,3)20(30-24(37)31-26(4,5)6)23(36)32-13-15-17(27(15,7)8)18(32)22(35)29-16(19(33)21(28)34)12-14-10-9-11-14/h14-18,20H,9-13H2,1-8H3,(H2,28,34)(H,29,35)(H2,30,31,37)/t15-,16?,17-,18-,20+/m0/s1
- InChIKey
- LHHCSNFAOIFYRV-DOVBMPENSA-N
|