| General Information of Drug (ID:
DR0222) |
| Drug Name |
Bosentan
|
| Synonyms |
Bosentan; Bosentan [USAN:INN:BAN]; Actelion; Ro 47-0203; Ro 47-0203/039; Ro-47-0203; Tracleer; XUL93R30K2; bosentanum; 147536-97-8; 4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-(2,2'-bipyrimidin)-4-yl)benzenesulfornamide; 4-tert-butyl-N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-pyrimidin-2-ylpyrimidin-4-yl]benzenesulfonamide; C27H29N5O6S; CHEBI:51450; CHEMBL957; UNII-XUL93R30K2; p-tert-Butyl-N-(6-(2-hydroxyethoxy)-5-(o-methoxyphenoxy)-2-(2-pyrimidinyl)-4-pyrimidinyl)benzenesulfonamide
|
| Indication |
Pulmonary hypertension
[ICD11: BB01]
|
Approved
|
[1]
|
| Structure |
|
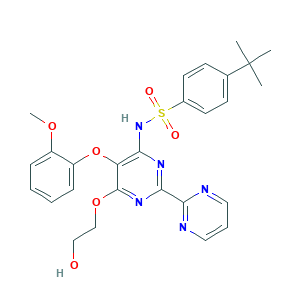 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
551.6 |
Topological Polar Surface Area |
154 |
| Heavy Atom Count |
39 |
Rotatable Bond Count |
11 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
11 |
| Cross-matching ID |
- PubChem CID
- 104865
- PubChem SID
-
7978805
; 10233650
; 12014838
; 14763764
; 44434454
; 46507154
; 47285934
; 48179783
; 49831038
; 49881484
; 50112694
; 50840650
; 51091861
; 53787963
; 56464382
; 57337889
; 85209778
; 92309270
; 92714194
; 93581179
; 93619683
; 103250068
; 103956688
; 104179184
; 104373115
; 118855338
; 125341362
; 126592988
; 126624339
; 126655593
; 126671144
; 127344134
; 127344135
; 127344136
; 127963291
; 131299472
; 134337358
; 134339787
; 135143300
; 135723687
; 137003210
; 142094009
; 143493318
; 144115748
; 144205997
; 151991482
; 152237729
; 152258948
; 160647793
; 160963904
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U4CE
- Formula
- C27H29N5O6S
- Canonical SMILES
- CC(C)(C)C1=CC=C(C=C1)S(=O)(=O)NC2=C(C(=NC(=N2)C3=NC=CC=N3)OCCO)OC4=CC=CC=C4OC
- InChI
- 1S/C27H29N5O6S/c1-27(2,3)18-10-12-19(13-11-18)39(34,35)32-23-22(38-21-9-6-5-8-20(21)36-4)26(37-17-16-33)31-25(30-23)24-28-14-7-15-29-24/h5-15,33H,16-17H2,1-4H3,(H,30,31,32)
- InChIKey
- GJPICJJJRGTNOD-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


