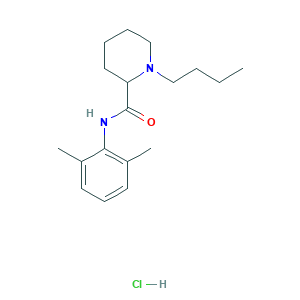
| Synonyms |
Bupivacaine (hydrochloride); Bupivacaine HCL; Bupivacaine hydrochloride; Bupivacaine hydrochloride [JAN]; CHEBI:31322; EINECS 241-917-8; Marcain; Marcaine Spinal; Vivacaine; Anekain; Bupivacaine; Bloqueina; Bupivacaina; Bupivacaina [INN-Spanish]; Bupivacaine HCL KIT; Bupivacainum; Bupivacainum [INN-Latin]; Carbostesin; DL-Bupivacaine; Marcaine; Win 11318; bupivacaine; bupivacaine base; cBupivacaine; dl-1-Butyl-2',6'-pipecoloxylidide; (+-)-Bupivacaine; (+/-)-Bupivacaine; 1-Butyl-2',6'-pipecoloxylidide; 1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide; 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide; 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-; 2180-92-9; 38396-39-3; (+-)-Bupivacaine hydrochloride; 1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide monohydrochloride; 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide hydrochloride; 14252-80-3; 18010-40-7; 2',6'-Pipecoloxylidide, 1-butyl-, hydrochloride; 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, monohydrochloride; AH-2250
|
| Cross-matching ID |
- PubChem CID
- 64737
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0A0FL
- Formula
- C18H29ClN2O
- Canonical SMILES
- CCCCN1CCCCC1C(=O)NC2=C(C=CC=C2C)C.Cl
- InChI
- 1S/C18H28N2O.ClH/c1-4-5-12-20-13-7-6-11-16(20)18(21)19-17-14(2)9-8-10-15(17)3;/h8-10,16H,4-7,11-13H2,1-3H3,(H,19,21);1H
- InChIKey
- SIEYLFHKZGLBNX-UHFFFAOYSA-N
|