Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0321) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Ciclosporin
|
|||||
| Synonyms |
Ciclosporin; Ciclosporin (Ciclosporin A); Ciclosporina; Ciclosporina [INN-Spanish]; Ciclosporine; Ciclosporine [INN-French]; Ciclosporinum; Ciclosporinum [INN-Latin]; Cipol N; Consupren; Cyclosporin; Cyclosporine A; Equoral; Antibiotic S 7481F1; Gengraf; Neoplanta; Neoral; Ramihyphin A; Restasis; S-Neoral; Sandimmun; Sandimmun Neoral; Sandimmune; Sang 35; Sang-35; SangCyA; Sigmasporin Microoral; cyclosporin A; cyclosporine; 59865-13-3; 83HN0GTJ6D; CHEBI:4031; CSA; DSSTox_CID_365; DSSTox_RID_75541; MFCD00274558; MLS001333756; UNII-83HN0GTJ6D
|
|||||
| Indication | Viral hepatitis [ICD11: 1E51] | Approved | [1] | |||
| Structure |
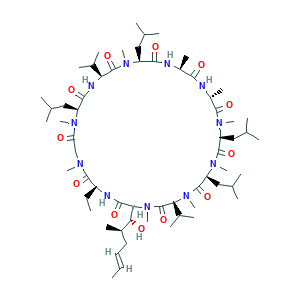 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 1202.6 | Topological Polar Surface Area | 279 | ||
| Heavy Atom Count | 85 | Rotatable Bond Count | 15 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 12 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

