Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0400) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Dabigatran etexilate
|
|||||
| Synonyms |
Dabigatran etexilate (USAN/INN); EBD35035; NCGC00262929-01; Pradaxa (TN); SCHEMBL505829; STL450990; STL483396; Tox21_113924; cc-72; ethyl N-[(2-{[(4-{N'-[(hexyloxy)carbonyl]carbamimidoyl}phenyl)amino]methyl}-1-methyl-1H-benzimidazol-5-yl)carbonyl]-N-pyridin-2-yl-beta-alaninate; s2154; Dabigatran; I0VM4M70GC; N-[(2-{[(4-Carbamimidoylphenyl)amino]methyl}-1-Methyl-1h-Benzimidazol-5-Yl)carbonyl]-N-Pyridin-2-Yl-Beta-Alanine; Q-102529; UNII-I0VM4M70GC; 211914-51-1; 3-[[2-[(4-carbamimidoylanilino)methyl]-1-methylbenzimidazole-5-carbonyl]-pyridin-2-ylamino]propanoic acid; 3-[[2-[[(4-CARBAMIMIDOYLPHENYL)AMINO]METHYL]-1-METHYL-BENZOIMIDAZOLE-5-CARBONYL]-PYRIDIN-2-YL-AMINO]PROPANOIC ACID; BIBR 953; BIBR 953 (Dabigatran, Pradaxa); BIBR-953; BIBR953; C25H25N7O3; CHEBI:70752; CHEMBL48361; 211915-06-9; A815191; AB01274780-01; AB01274780_02; BDBM50432209; BIBR-1048; BIBR1048; CAS-211915-06-9; D07144; DSSTox_CID_31470; DSSTox_GSID_57681; DSSTox_RID_97355
|
|||||
| Indication | Venous thromboembolism [ICD11: BD72] | Approved | [1] | |||
| Structure |
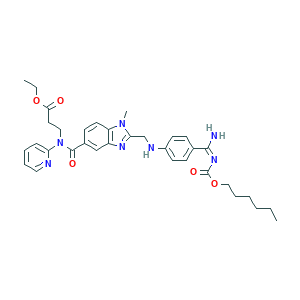 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 627.7 | Topological Polar Surface Area | 154 | ||
| Heavy Atom Count | 46 | Rotatable Bond Count | 17 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 8 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Dabigatran Etexilate was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | Pharmacogenomics of novel direct oral anticoagulants: newly identified genes and genetic variants. J Pers Med. 2019 Jan 17;9(1). pii: E7. | |||||
| 3 | Dabigatran acylglucuronide, the major human metabolite of dabigatran: in vitro formation, stability, and pharmacological activity. Drug Metab Dispos. 2010 Sep;38(9):1567-75. | |||||
| 4 | The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans | |||||
| 5 | Conventional liquid chromatography/triple quadrupole mass spectrometry based metabolite identification and semi-quantitative estimation approach in the investigation of in vitro dabigatran etexilate metabolism. Anal Bioanal Chem. 2013 Feb;405(5):1695-704. | |||||
| 6 | Identification of carboxylesterase-dependent dabigatran etexilate hydrolysis | |||||
| 7 | DrugBank(Pharmacology-Metabolism): Dabigatran etexilate | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

