| General Information of Drug (ID:
DR0407) |
| Drug Name |
Dacomitinib
|
| Synonyms |
Dacomitinib; Dacomitinib (PF299804, PF299); Dacomitinib [USAN:INN]; J-500784; PF 00299804-03; PF-00299804; PF-00299804-03; PF299804; dacomitinibum; pf00299804; (2E)-N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-methoxy-6-quinazolinyl]-4-(1-piperidinyl)-2-butenamide; (2e)-N-{4-[(3-Chloro-4-Fluorophenyl)amino]-7-Methoxyquinazolin-6-Yl}-4-(Piperidin-1-Yl)but-2-Enamide; (E)-N-(4-((3-chloro-4-fluorophenyl)aMino)-7-Methoxyquinazolin-6-yl)-4-(piperidin-1-yl)but-2-enaMide; 1110813-31-4; 2XJX250C20; C24H25ClFN5O2; PF299; UNII-2XJX250C20
|
| Indication |
Lung cancer
[ICD11: 2C25]
|
Approved
|
[1]
|
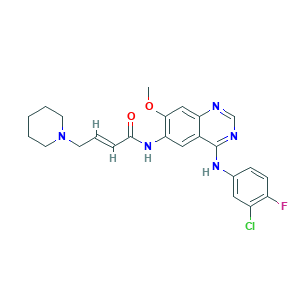
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
469.9 |
Topological Polar Surface Area |
79.4 |
| Heavy Atom Count |
33 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 11511120
- PubChem SID
-
16613030
; 23640600
; 74609599
; 135263265
; 135626635
; 136340260
; 136349571
; 136367702
; 140977763
; 152159560
; 152258509
; 160647344
; 162011542
; 162038136
; 162205175
; 164041746
; 172918917
; 174505192
; 174526192
; 178103994
; 185997026
; 189622863
; 194690240
; 198956821
; 223382341
; 223705276
; 252109876
; 252216044
; 252451737
; 252451797
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06XXH
- Formula
- C24H25ClFN5O2
- Canonical SMILES
- COC1=C(C=C2C(=C1)N=CN=C2NC3=CC(=C(C=C3)F)Cl)NC(=O)C=CCN4CCCCC4
- InChI
- 1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+
- InChIKey
- LVXJQMNHJWSHET-AATRIKPKSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


