| Synonyms |
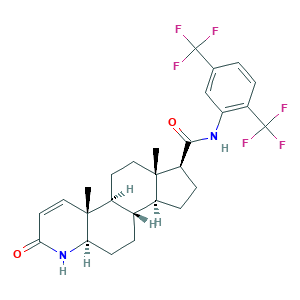
Dutasteride; Dutasteride, 99%; GG 745; GG-745; GI 198745; GI-198745; GI198745; Avodart; Avolve; O0J6XJN02I; (1S,3aS,3bS,5aR,9aR,9bS,11aS)-N-[2,5-bis(trifluoromethyl)phenyl]-9a,11a-dimethyl-7-oxo-1,2,3,3a,3b,4,5,5a,6,9b,10,11-dodecahydroindeno[5,4-f]quinoline-1-carboxamide; (5alpha,17beta)-N-(2,5-Bis(trifluoromethyl)phenyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide; 164656-23-9; CHEBI:521033; CHEMBL1200969; UNII-O0J6XJN02I
|
| Cross-matching ID |
- PubChem CID
- 6918296
- PubChem SID
-
12014955
; 14836607
; 14909902
; 17194796
; 17397907
; 43529678
; 46504830
; 53787436
; 57371871
; 71824992
; 79317270
; 81092907
; 85709776
; 91145910
; 91612910
; 99437024
; 103770952
; 104179082
; 114787740
; 118046363
; 124757077
; 125163881
; 125311722
; 126596711
; 131343417
; 135109202
; 135684130
; 136183917
; 136367959
; 136375517
; 136980377
; 137005555
; 137181708
; 142643797
; 144115926
; 144205752
; 152059555
; 152063850
; 162036214
; 162175331
; 163797282
; 165698504
; 172093501
; 174477509
; 174527666
; 175265390
; 176484953
; 179150039
; 187051760
; 210274902
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0A9YA
- Formula
- C27H30F6N2O2
- Canonical SMILES
- CC12CCC3C(C1CCC2C(=O)NC4=C(C=CC(=C4)C(F)(F)F)C(F)(F)F)CCC5C3(C=CC(=O)N5)C
- InChI
- 1S/C27H30F6N2O2/c1-24-11-9-17-15(4-8-21-25(17,2)12-10-22(36)35-21)16(24)6-7-19(24)23(37)34-20-13-14(26(28,29)30)3-5-18(20)27(31,32)33/h3,5,10,12-13,15-17,19,21H,4,6-9,11H2,1-2H3,(H,34,37)(H,35,36)/t15-,16-,17-,19+,21+,24-,25+/m0/s1
- InChIKey
- JWJOTENAMICLJG-QWBYCMEYSA-N
|