| Cross-matching ID |
- PubChem CID
- 6442177
- PubChem SID
-
12014881
; 14767667
; 14865574
; 50044230
; 50112765
; 56311446
; 56312241
; 56312580
; 56313164
; 91613187
; 104178966
; 134338463
; 135156085
; 136340120
; 136929859
; 137140572
; 139754909
; 143493384
; 144206063
; 151990396
; 152236848
; 152258132
; 160645716
; 160646971
; 162189189
; 174527790
; 175265707
; 177748738
; 179150022
; 203355779
; 226396389
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0K3QS
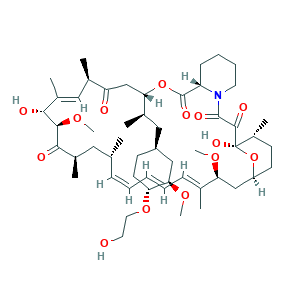
- Formula
- C53H83NO14
- Canonical SMILES
- CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC
- InChI
- 1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1
- InChIKey
- HKVAMNSJSFKALM-GKUWKFKPSA-N
|