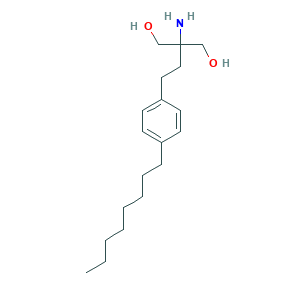
| Synonyms |
Fingolimod; Fingolimod (INN); Fingolimod [INN]; fingolimodum; 1,3-Propanediol, 2-amino-2-[2-(4-octylphenyl)ethyl]-; 162359-55-9; 2-Amino-2-(4-octylphenethyl)propane-1,3-diol; 2-Amino-2-[2-(4-octylphenyl)ethyl]-1,3-propandiol; 2-Amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol; 2-Amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol; 2-amino-2-(2-(4-octylphenyl)ethyl)-1,3-propanediol; 2-amino-2-(2-(4-octylphenyl)ethyl)propane-1,3-diol; 3QN8BYN5QF; C19H33NO2; CHEBI:63115; CHEMBL314854; UNII-3QN8BYN5QF
|
| Cross-matching ID |
- PubChem CID
- 107970
- PubChem SID
-
7980354
; 10627340
; 11538030
; 14800795
; 44436888
; 50070757
; 52231945
; 91613352
; 103298437
; 104045114
; 104380165
; 112629137
; 124899206
; 125335078
; 126522750
; 126659901
; 126665797
; 128394615
; 134339282
; 135204658
; 135626722
; 135650261
; 137003753
; 140236536
; 152344348
; 160820803
; 162221744
; 163091250
; 163838506
; 164834614
; 174006739
; 175268765
; 175426965
; 175427125
; 184527777
; 204414213
; 210274752
; 210280385
; 223660888
; 223770775
; 226398832
; 242078815
; 249836440
; 251873579
; 251916753
; 251917992
; 252214240
; 252433026
; 252478872
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07UHS
- Formula
- C19H33NO2
- Canonical SMILES
- CCCCCCCCC1=CC=C(C=C1)CCC(CO)(CO)N
- InChI
- 1S/C19H33NO2/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-22/h9-12,21-22H,2-8,13-16,20H2,1H3
- InChIKey
- KKGQTZUTZRNORY-UHFFFAOYSA-N
|