| General Information of Drug (ID:
DR0968) |
| Drug Name |
Lomefloxacin
|
| Synonyms |
Lomefloxacine; Lomefloxacine [French]; Lomefloxacino; Lomefloxacino [Spanish]; Lomefloxacinum; Lomefloxacinum [Latin]; SC 47111A; DM 10 (bactericide); SC-47111A; lomefloxacin; (+-)-1-Ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1,4-Dihydro-6,8-difluoro-1-ethyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-ethyl-6,8-difluoro-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; 98079-51-7; BRN 4210041; CCRIS 6305; CHEBI:116278; DM-10; LFLX
|
| Indication |
Conjunctivitis
[ICD11: 9A60]
|
Approved
|
[1]
|
| Structure |
|
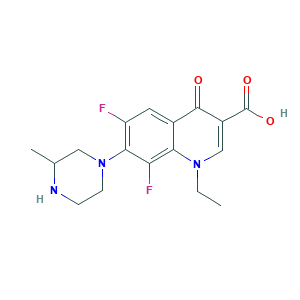 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
351.35 |
Topological Polar Surface Area |
72.9 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 3948
- PubChem SID
-
9289
; 602955
; 5037068
; 7849377
; 7979788
; 8152478
; 11335540
; 11360779
; 11364325
; 11366887
; 11369449
; 11372706
; 11373775
; 11377611
; 11461751
; 11466266
; 11467386
; 11484587
; 11485972
; 11488512
; 11491309
; 11491973
; 11495245
; 14851991
; 24278514
; 29223062
; 46508499
; 47291062
; 47588922
; 47588923
; 47736397
; 47736398
; 48035031
; 48110376
; 48334411
; 48334412
; 49698401
; 49835841
; 50042514
; 50111115
; 50122921
; 50122922
; 50203310
; 56313756
; 57322062
; 85321506
; 85788434
; 90340951
; 92309292
; 103178831
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02KOF
- Formula
- C17H19F2N3O3
- Canonical SMILES
- CCN1C=C(C(=O)C2=CC(=C(C(=C21)F)N3CCNC(C3)C)F)C(=O)O
- InChI
- 1S/C17H19F2N3O3/c1-3-21-8-11(17(24)25)16(23)10-6-12(18)15(13(19)14(10)21)22-5-4-20-9(2)7-22/h6,8-9,20H,3-5,7H2,1-2H3,(H,24,25)
- InChIKey
- ZEKZLJVOYLTDKK-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


