| Synonyms |
Midostaurin; Midostaurin (PKC412); Midostaurin (USAN/INN); Midostaurin [USAN:INN]; N-Benzoylstaurosporine; PKC 412; PKC-412; PKC412; Benzoylstaurosporine; RYDAPT; Rydapt (TN); midostaurin-pkc412; 120685-11-2; 4'-N-Benzoylstaurosporine; CGP 41231; CGP-41251; CHEBI:63452; CHEMBL608533; Cgp 41 251; Cgp 41251; ID912S5VON; N-[(5S,6R,7R,9R)-6-methoxy-5-methyl-14-oxo-6,7,8,9,15,16-hexahydro-5H,14H-5,9-epoxy-4b,9a,15-triazadibenzo[b,h]cyclonona[1,2,3,4-jkl]cyclopenta[e]-as-indacen-7-yl]-N-methylbenzamide; UNII-ID912S5VON
|
| Cross-matching ID |
- PubChem CID
- 9829523
- PubChem SID
-
14788708
; 14935507
; 22395186
; 44927646
; 47206756
; 53786846
; 57373453
; 79311635
; 99302777
; 103734272
; 123105168
; 124659175
; 124950161
; 134348392
; 135061643
; 135610396
; 137241200
; 143298037
; 172918683
; 174006478
; 177748492
; 178102329
; 179149698
; 184812273
; 198965438
; 210274665
; 210280297
; 223656331
; 233822082
; 241376205
; 248897758
; 249617730
; 252156932
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07NVU
- Formula
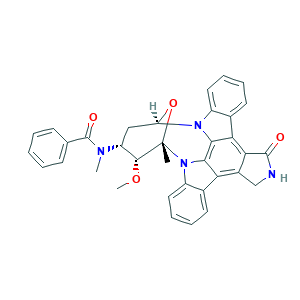
- C35H30N4O4
- Canonical SMILES
- CC12C(C(CC(O1)N3C4=CC=CC=C4C5=C6C(=C7C8=CC=CC=C8N2C7=C53)CNC6=O)N(C)C(=O)C9=CC=CC=C9)OC
- InChI
- 1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1
- InChIKey
- BMGQWWVMWDBQGC-IIFHNQTCSA-N
|