| Cross-matching ID |
- PubChem CID
- 24958200
- PubChem SID
-
56256045
; 57487185
; 103749792
; 104150587
; 135265184
; 135626858
; 136340151
; 136349580
; 136367711
; 136946461
; 137232464
; 138192109
; 144116229
; 152258260
; 160647096
; 160829563
; 162038124
; 162113109
; 163094037
; 163394941
; 163843781
; 174007122
; 186007055
; 189561499
; 198976487
; 202829003
; 204430248
; 223617456
; 227634434
; 247056359
; 249752894
; 252080192
; 252166487
; 252227886
; 252300335
; 252462459
- CAS Number
-
- TTD Drug ID
- D00BMF
- Formula
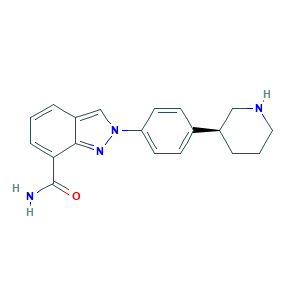
- C19H20N4O
- Canonical SMILES
- C1CC(CNC1)C2=CC=C(C=C2)N3C=C4C=CC=C(C4=N3)C(=O)N
- InChI
- 1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1
- InChIKey
- PCHKPVIQAHNQLW-CQSZACIVSA-N
|