| General Information of Drug (ID:
DR1188) |
| Drug Name |
Olaparib
|
| Synonyms |
Olaparib; Olaparib (AZD2281, Ku-0059436); WOH1JD9AR8; KU-0059436; KU-59436; Lynparza; 1-(Cyclopropylcarbonyl)-4-[5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluorobenzoyl]piperazine; 4-(3-(4-(Cyclopropanecarbonyl)piperazine-1-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one; 4-[3-(4-Cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluoro-benzyl]-2H-phthalazin-1-one; 763113-22-0; AZD 2281; AZD-2281; AZD2281; AZD2281(olaparib); CHEBI:83766; MFCD13185161; UNII-WOH1JD9AR8
|
| Indication |
Ovarian cancer
[ICD11: 2C73]
|
Approved
|
[1]
|
| Structure |
|
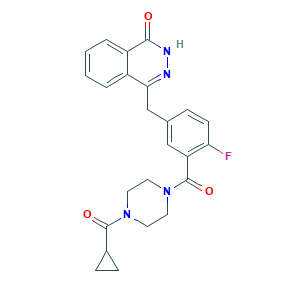 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
434.5 |
Topological Polar Surface Area |
82.1 |
| Heavy Atom Count |
32 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 23725625
- PubChem SID
-
47209066
; 56053773
; 57299275
; 85197660
; 93581006
; 99436961
; 103605183
; 109692964
; 117670453
; 118049496
; 121277945
; 123051082
; 124490470
; 124756970
; 124947876
; 125163775
; 125312522
; 125415525
; 126582065
; 126626885
; 126644879
; 126664211
; 126666996
; 126738835
; 131407195
; 131465127
; 134213960
; 134222742
; 134338825
; 134339140
; 134346179
; 134964396
; 135261055
; 135697767
; 135708109
; 135723596
; 135727477
; 136368068
; 136377786
; 136895641
; 136920280
; 137005640
; 137255348
; 141853509
; 143499369
; 144115666
; 152237700
; 152258100
; 152344243
; 160646939
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0J9HW
- Formula
- C24H23FN4O3
- Canonical SMILES
- C1CC1C(=O)N2CCN(CC2)C(=O)C3=C(C=CC(=C3)CC4=NNC(=O)C5=CC=CC=C54)F
- InChI
- 1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30)
- InChIKey
- FDLYAMZZIXQODN-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


